Structuration in the interface of direct and reversed micelles of sucrose esters, studied by fluorescent techniques
- PMID: 25905632
- PMCID: PMC4408079
- DOI: 10.1371/journal.pone.0123669
Structuration in the interface of direct and reversed micelles of sucrose esters, studied by fluorescent techniques
Abstract
Background: Reactors found in nature can be described as micro-heterogeneous systems, where media involved in each micro-environment can behave in a markedly different way compared with the properties of the bulk solution. The presence of water molecules in micro-organized assemblies is of paramount importance for many chemical processes, ranging from biology to environmental science. Self-organized molecular assembled systems are frequently used to study dynamics of water molecules because are the simplest models mimicking biological membranes. The hydrogen bonds between sucrose and water molecules are described to be stronger (or more extensive) than the ones between water molecules themselves. In this work, we studied the capability of sucrose moiety, attached to alkyl chains of different length, as a surface blocking agent at the water-interface and we compared its properties with those of polyethylenglycol, a well-known agent used for this purposes. Published studies in this topic mainly refer to the micellization process and the stability of mixed surfactant systems using glycosides. We are interested in the effect induced by the presence of sucrose monoesters at the interface (direct and reverse micelles) and at the palisade (mixtures with Triton X-100). We believe that the different functional group (ester), the position of alkyl chain (6-O) and the huge capability of sucrose to interact with water will dramatically change the water structuration at the interface and at the palisade, generating new possibilities for technological applications of these systems.
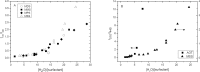
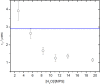
Results: Our time resolved and steady state fluorescence experiments in pure SEs micelles show that sucrose moieties are able to interact with a high number of water molecules promoting water structuration and increased viscosity. These results also indicate that the barrier formed by sucrose moieties on the surface of pure micelles is more effective than the polyoxyethylene palisade of Triton X-100. The fluorescence quenching experiments of SEs at the palisade of Triton X-100 micelles indicate a blocking effect dependent on the number of methylene units present in the hydrophobic tail of the surfactant. A remarkable blocking effect is observed when there is a match in size between the hydrophobic regions forming the apolar core (lauryl SE/ Triton X-100). This blocking effect disappears when a mismatch in size between hydrophobic tails, exists due to the disturbing effect on the micelle core.
Conflict of interest statement
Figures






Similar articles
-
Sucrose monoester micelles size determined by Fluorescence Correlation Spectroscopy (FCS).PLoS One. 2011;6(12):e29278. doi: 10.1371/journal.pone.0029278. Epub 2011 Dec 28. PLoS One. 2011. PMID: 22216230 Free PMC article.
-
PFGSE-NMR study of the self-diffusion of sucrose fatty acid monoesters in water.J Colloid Interface Sci. 2005 Jun 1;286(1):360-8. doi: 10.1016/j.jcis.2004.12.010. J Colloid Interface Sci. 2005. PMID: 15848439
-
Solvation dynamics in triton-X-100 and triton-X-165 micelles: effect of micellar size and hydration.J Chem Phys. 2004 Sep 22;121(12):6026-33. doi: 10.1063/1.1784774. J Chem Phys. 2004. PMID: 15367031
-
Complementary use of simulations and molecular-thermodynamic theory to model micellization.Langmuir. 2006 Feb 14;22(4):1500-13. doi: 10.1021/la052042c. Langmuir. 2006. PMID: 16460068 Review.
-
Large organized surface domains self-assembled from nonpolar amphiphiles.Acc Chem Res. 2012 Apr 17;45(4):514-24. doi: 10.1021/ar200178a. Epub 2011 Dec 21. Acc Chem Res. 2012. PMID: 22185721 Review.
References
-
- Borsarelli CD, Braslavsky SE. Nature of the water structure inside the pools of reverse micelles sensed by laser-induced optoacoustic spectroscopy. J Phys Chem B. 1997; 101: 6036–6042.
-
- Kumbhakar M, Nath S, Mukherjee T, Pal H. Solvation dynamics in triton-X-100 and triton-X-165 micelles: Effect of micellar size and hydration. J Chem Phys. 2004; 121: 6026–6033. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

