Safety of intracoronary infusion of 20 million C-kit positive human cardiac stem cells in pigs
- PMID: 25905721
- PMCID: PMC4408046
- DOI: 10.1371/journal.pone.0124227
Safety of intracoronary infusion of 20 million C-kit positive human cardiac stem cells in pigs
Abstract
Background: There is mounting interest in using c-kit positive human cardiac stem cells (c-kit(pos) hCSCs) to repair infarcted myocardium in patients with ischemic cardiomyopathy. A recent phase I clinical trial (SCIPIO) has shown that intracoronary infusion of 1 million hCSCs is safe. Higher doses of CSCs may provide superior reparative ability; however, it is unknown if doses >1 million cells are safe. To address this issue, we examined the effects of 20 million hCSCs in pigs.
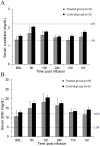
Methods: Right atrial appendage samples were obtained from patients undergoing cardiac surgery. The tissue was processed by an established protocol with eventual immunomagnetic sorting to obtain in vitro expanded hCSCs. A cumulative dose of 20 million cells was given intracoronarily to pigs without stop flow. Safety was assessed by measurement of serial biomarkers (cardiac: troponin I and CK-MB, renal: creatinine and BUN, and hepatic: AST, ALT, and alkaline phosphatase) and echocardiography pre- and post-infusion. hCSC retention 30 days after infusion was quantified by PCR for human genomic DNA. All personnel were blinded as to group assignment.
Results: Compared with vehicle-treated controls (n=5), pigs that received 20 million hCSCs (n=9) showed no significant change in cardiac function or end organ damage (assessed by organ specific biomarkers) that could be attributed to hCSCs (P>0.05 in all cases). No hCSCs could be detected in left ventricular samples 30 days after infusion.
Conclusions: Intracoronary infusion of 20 million c-kit positive hCSCs in pigs (equivalent to ~40 million hCSCs in humans) does not cause acute cardiac injury, impairment of cardiac function, or liver and renal injury. These results have immediate translational value and lay the groundwork for using doses of CSCs >1 million in future clinical trials. Further studies are needed to ascertain whether administration of >1 million hCSCs is associated with greater efficacy in patients with ischemic cardiomyopathy.
Conflict of interest statement
Figures









References
-
- Beltrami AP, Barlucchi L, Torella D, Baker M, Limana F, Chimenti S, et al. Adult cardiac stem cells are multipotent and support myocardial regeneration. Cell. 2003;114: 763–776. - PubMed
-
- Meluzin J, Mayer J, Groch L, Janousek S, Hornacek I, Hlinomaz O, et al. Autologous transplantation of mononuclear bone marrow cells in patients with acute myocardial infarction: the effect of the dose of transplanted cells on myocardial function. Am Heart J. 2006;152: 975 e979–915. - PubMed
-
- Katritsis DG, Sotiropoulou PA, Karvouni E, Karabinos I, Korovesis S, Perez SA, et al. Transcoronary transplantation of autologous mesenchymal stem cells and endothelial progenitors into infarcted human myocardium. Catheter Cardiovasc Interv. 2005;65: 321–329. - PubMed
-
- Gao LR, Pei XT, Ding QA, Chen Y, Zhang NK, Chen HY, et al. A critical challenge: dosage-related efficacy and acute complication intracoronary injection of autologous bone marrow mesenchymal stem cells in acute myocardial infarction. Int J Cardiol. 2013;168: 3191–3199. 10.1016/j.ijcard.2013.04.112 - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

