Synthesis of Morphinan Alkaloids in Saccharomyces cerevisiae
- PMID: 25905794
- PMCID: PMC4408053
- DOI: 10.1371/journal.pone.0124459
Synthesis of Morphinan Alkaloids in Saccharomyces cerevisiae
Abstract
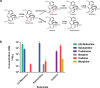
Morphinan alkaloids are the most powerful narcotic analgesics currently used to treat moderate to severe and chronic pain. The feasibility of morphinan synthesis in recombinant Saccharomyces cerevisiae starting from the precursor (R,S)-norlaudanosoline was investigated. Chiral analysis of the reticuline produced by the expression of opium poppy methyltransferases showed strict enantioselectivity for (S)-reticuline starting from (R,S)-norlaudanosoline. In addition, the P. somniferum enzymes salutaridine synthase (PsSAS), salutaridine reductase (PsSAR) and salutaridinol acetyltransferase (PsSAT) were functionally co-expressed in S. cerevisiae and optimization of the pH conditions allowed for productive spontaneous rearrangement of salutaridinol-7-O-acetate and synthesis of thebaine from (R)-reticuline. Finally, we reconstituted a 7-gene pathway for the production of codeine and morphine from (R)-reticuline. Yeast cell feeding assays using (R)-reticuline, salutaridine or codeine as substrates showed that all enzymes were functionally co-expressed in yeast and that activity of salutaridine reductase and codeine-O-demethylase likely limit flux to morphine synthesis. The results of this study describe a significant advance for the synthesis of morphinans in S. cerevisiae and pave the way for their complete synthesis in recombinant microbes.
Conflict of interest statement
Figures



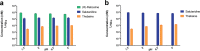
References
-
- Millgate AG, Pogson BJ, Wilson IW, Kutchan TM, Zenk MH, Gerlach WL, et al. Analgesia: morphine-pathway block in top1 poppies. Nature. 2004;431: 413–414. - PubMed
-
- INCB Supply of opiate raw materials and demand for opiates for medical and scientific purposes. Estim World Requir 2014—Stat 2012: 2013;3: 92–10. Available: http://www.incb.org/documents/Narcotic-Drugs/Technical-Publications/2012.... Accessed 30 September 2014.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

