Neural regulation of hematopoiesis, inflammation, and cancer
- PMID: 25905810
- PMCID: PMC4416657
- DOI: 10.1016/j.neuron.2015.01.026
Neural regulation of hematopoiesis, inflammation, and cancer
Abstract
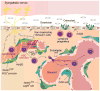
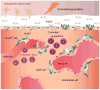
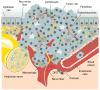
Although the function of the autonomic nervous system (ANS) in mediating the flight-or-fight response was recognized decades ago, the crucial role of peripheral innervation in regulating cell behavior and response to the microenvironment has only recently emerged. In the hematopoietic system, the ANS regulates stem cell niche homeostasis and regeneration and fine-tunes the inflammatory response. Additionally, emerging data suggest that cancer cells take advantage of innervating neural circuitry to promote their progression. These new discoveries outline the need to redesign therapeutic strategies to target this underappreciated stromal constituent. Here, we review the importance of neural signaling in hematopoietic homeostasis, inflammation, and cancer.
Copyright © 2015 Elsevier Inc. All rights reserved.
Figures



References
-
- ARAI F, HIRAO A, OHMURA M, SATO H, MATSUOKA S, TAKUBO K, ITO K, KOH GY, SUDA T. Tie2/Angiopoietin-1 Signaling Regulates Hematopoietic Stem Cell Quiescence in the Bone Marrow Niche. Cell. 2004;118:149–161. - PubMed
-
- ARRANZ L, SANCHEZ-AGUILERA A, MARTIN-PEREZ D, ISERN J, LANGA X, TZANKOV A, LUNDBERG P, MUNTION S, TZENG Y-S, LAI D-M, SCHWALLER J, SKODA RC, MENDEZ-FERRER S. Neuropathy of haematopoietic stem cell niche is essential for myeloproliferative neoplasms. Nature. 2014 doi: 10.1038/nature13383. Advanced online piblication 06/22. - DOI - PubMed
-
- ASADA N, KATAYAMA Y, SATO M, MINAGAWA K, WAKAHASHI K, KAWANO H, KAWANO Y, SADA A, IKEDA K, MATSUI T, TANIMOTO M. Matrix-embedded osteocytes regulate mobilization of hematopoietic stem/progenitor cells. Cell Stem Cell. 2013;12:737–47. - PubMed
-
- AYALA GE, DAI H, POWELL M, LI R, DING Y, WHEELER TM, SHINE D, KADMON D, THOMPSON T, MILES BJ, ITTMANN MM, ROWLEY D. Cancer-related axonogenesis and neurogenesis in prostate cancer. Clin Cancer Res. 2008;14:7593–603. - PubMed
-
- AYALA GE, DAI H, TAHIR SA, LI R, TIMME T, ITTMANN M, FROLOV A, WHEELER TM, ROWLEY D, THOMPSON TC. Stromal antiapoptotic paracrine loop in perineural invasion of prostatic carcinoma. Cancer Res. 2006;66:5159–64. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

