Diffuse Cystic Lung Disease. Part I
- PMID: 25906089
- PMCID: PMC5442966
- DOI: 10.1164/rccm.201411-2094CI
Diffuse Cystic Lung Disease. Part I
Abstract
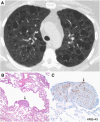
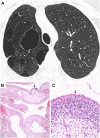
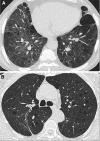
The diffuse cystic lung diseases (DCLDs) are a group of pathophysiologically heterogenous processes that are characterized by the presence of multiple spherical or irregularly shaped, thin-walled, air-filled spaces within the pulmonary parenchyma. Although the mechanisms of cyst formation remain incompletely defined for all DCLDs, in most cases lung remodeling associated with inflammatory or infiltrative processes results in displacement, destruction, or replacement of alveolar septa, distal airways, and small vessels within the secondary lobules of the lung. The DCLDs can be broadly classified according to underlying etiology as those caused by low-grade or high-grade metastasizing neoplasms, polyclonal or monoclonal lymphoproliferative disorders, infections, interstitial lung diseases, smoking, and congenital or developmental defects. In the first of a two-part series, we present an overview of the cystic lung diseases caused by neoplasms, infections, smoking-related diseases, and interstitial lung diseases, with a focus on lymphangioleiomyomatosis and pulmonary Langerhans cell histiocytosis.
Keywords: high-resolution computed tomography; lung cysts; lymphangioleiomyomatosis; pulmonary Langerhans cell histiocytosis; tuberous sclerosis.
Figures



References
Publication types
MeSH terms
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

