Preoperative ripening of the cervix before operative hysteroscopy
- PMID: 25906113
- PMCID: PMC11024390
- DOI: 10.1002/14651858.CD005998.pub2
Preoperative ripening of the cervix before operative hysteroscopy
Abstract
Background: Hysteroscopy is an operation in which the gynaecologist examines the uterine cavity using a small telescopic instrument (hysteroscope) inserted via the vagina and the cervix. Almost 50% of hysteroscopic complications are related to difficulty with cervical entry. Potential complications include cervical tears, creation of a false passage, perforation, bleeding, or simply difficulty in entering the internal os (between the cervix and the uterus) with the hysteroscope. These complications may possibly be reduced with adequate preparation of the cervix (cervical ripening) prior to hysteroscopy. Cervical ripening agents include oral or vaginal prostaglandin, which can be synthetic (e.g misoprostol) or natural (e.g. dinoprostone) and vaginal osmotic dilators, which can be naturally occurring (e.g. laminaria) or synthetic.
Objectives: To determine whether preoperative cervical preparation facilitates cervical dilatation and reduces the complications of operative hysteroscopy in women undergoing the procedure for any condition.
Search methods: In August 2014 we searched sources including the Menstrual Disorders and Subfertility Group (MDSG) Trials Register, Cochrane Central Register of Controlled Trials (CENTRAL), MEDLINE, EMBASE, PsycINFO, CINAHL, ClinicalTrials.gov and reference lists of relevant articles. We searched for published and unpublished studies in any language.
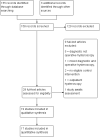
Selection criteria: Two review authors independently selected randomised controlled trials (RCTs) of cervical ripening agents used before operative hysteroscopy in pre- and postmenopausal women. Cervical ripening agents could be compared to each other, placebo or no treatment.
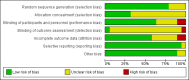
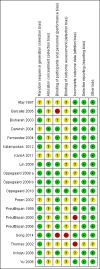
Data collection and analysis: Data extraction and quality assessment were conducted independently by two review authors. The primary review outcomes were effectiveness of cervical dilatation (defined as the proportion of women requiring mechanical cervical dilatation) and intraoperative complications. Secondary outcomes were mean time required to dilate the cervix, preoperative pain, cervical width, abandonment of the procedure, side effects of dilating agents and duration of surgery. We calculated odds ratios (ORs) for dichotomous outcomes and mean differences (MDs) for continuous outcomes, with 95% confidence intervals ( CIs). Data were statistically pooled where appropriate. Heterogeneity was assessed using the I(2) statistic. The overall quality of the evidence was assessed using GRADE methods.
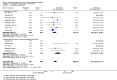
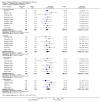
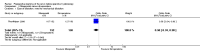
Main results: Nineteen RCTs with a total of 1870 participants were included. They compared misoprostol with no treatment or placebo, dinoprostone or osmotic dilators.Misoprostol was more effective for cervical dilatation than placebo or no intervention, with fewer women requiring mechanical dilatation (OR 0.08, 95% CI 0.04 to 0.16, five RCTs, 441 participants, I(2)=0%, moderate quality evidence). This suggests that in a population in which 80% of women undergoing hysteroscopy require mechanical dilatation without use of preoperative ripening agents, use of misoprostol will reduce the need for mechanical dilatation to between 14% and 39%. Misoprostol was associated with fewer intraoperative complications (OR 0.37, 95% CI 0.18 to 0.77, 12 RCTs, 901 participants, I(2)=0%, moderate quality evidence). This suggests that in a population in which 3% of women undergoing hysteroscopy experience intraoperative complications without use of preoperative ripening agents, use of misoprostol will reduce the risk of complications to 2% or less.When specific complications were considered, the misoprostol group had a lower rate of cervical laceration or tearing (OR 0.25, 95% CI 0.11 to 0.57, nine RCTS, 669 women, I(2)=0%, moderate quality evidence) or false track formation (OR 0.34, 95% CI 0.12 to 0.97, seven RCTs, 560 participants, I(2)=0%, moderate quality evidence). There was no evidence of a difference between the groups in rates of uterine perforation (0.42, 95% CI 0.13 to 1.38, seven RCTs, 455 participants, I(2)=0%, low quality evidence) or uterine bleeding (OR 0.51, 95% CI 0.10 to 2.49, four RCTs, 340 participants, I(2)=0%, low quality evidence). Some treatment side effects (mild abdominal pain, vaginal bleeding, and increased body temperature) were more common in the misoprostol group.Compared with dinoprostone, misoprostol was associated with more effective cervical dilatation, with fewer women requiring mechanical dilatation (OR 0.58; 95% CI 0.34 to 0.98; one RCT, 310 participants, low quality evidence) and with fewer intraoperative complications (OR 0.32; 95% CI 0.12 to 0.83, one RCT, 310 participants, low quality evidence). However treatment side effects were more common in the misoprostol arm.Compared to osmotic dilatation (laminaria), misoprostol was associated with less effective cervical dilatation, with more women in the misoprostol group requiring mechanical dilatation (OR 5.96, 95% CI 2.61 to 13.59, one RCT, 110 participants, low quality evidence). There was no evidence of a difference between misoprostol and osmotic dilators in intraoperative complication rates (OR 5.14, 95% CI 0.24 to 109.01, three RCTs, 354 participants, low quality evidence), with only two events reported altogether.The overall quality of the evidence ranged from low to moderate. The main limitations in the evidence were imprecision and poor reporting of study methods.
Authors' conclusions: There is moderate quality evidence that use of misoprostol for preoperative ripening of the cervix before operative hysteroscopy is more effective than placebo or no treatment and is associated with fewer intraoperative complications such as lacerations and false tracks. However misoprostol is associated with more side effects, including preoperative pain and vaginal bleeding. There is low quality evidence to suggest that misoprostol has fewer intraoperative complications and is more effective than dinoprostone.There is also low quality evidence to suggest that laminaria may be more effective than misoprostol, with uncertain effects for complication rates. However the possible benefits of laminaria need to be weighed against the inconvenience of its insertion and retention for one to two days.
Conflict of interest statement
None
Figures

















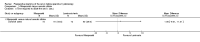
Update of
- doi: 10.1002/14651858.CD005998
References
References to studies included in this review
Atay 1997 {published data only}
-
- Atay V, Duru NK, Pabuccu R, Ergun A, Tokac G, Aydin BA. Vaginal misoprostol for cervical dilatation before operative office hysteroscopy. Gynaecological Endoscopy 1997;6:47‐9.
Barcaite 2005 {published data only}
-
- Barcaite E, Bartusevicius A, Railaite DR, Nadisauskiene R. Vaginal misoprostol for cervical priming before hysteroscopy in perimenopausal and postmenopausal women. International Journal of Gynaecology and Obstetrics 2005;91:141‐5. - PubMed
Bisharah 2003 {published data only}
-
- Bisharah M, Al‐Fozan H, Tulandi T. A randomized trial of sublingual misoprostol for cervical priming before hysteroscopy. The Journal of American Association of Gynecologic Laparoscopists 2003;10:390‐1. - PubMed
Darwish 2004 {published data only}
-
- Darwish AM, Ahmad AM, Mohammad AM. Cervical priming prior to operative hysteroscopy: a randomized comparison of laminaria versus misoprostol. Human Reproduction 2004;19:2391‐4. - PubMed
Fernandez 2004 {published data only}
-
- Fernandez H, Alby JD, Tournoux C, Chauveaud‐Lambling A, et al. Vaginal misoprostol for cervical ripening before operative hysteroscopy in pre‐menopausal women:a double ‐blind,placebo‐controlled trial with three dose regimens. Human Reproduction 2004;19:1618‐21. - PubMed
Kalampokas 2012 {published data only}
-
- Kalampokas E, Sofoudis C, Antonogeorgos G, Panoulis K, et al. A randomised controlled trial for cervical priming using vaginal misoprostol prior to hysteroscopy in women who have only undergone cesarean section. Archives of Gynecology and Obstetrics 2012;286(4):2374‐7. - PubMed
Kant A 2011 {published data only}
Lin 2009 {published data only}
-
- Lin YH, Hwang JL, Seow KM, Huang LW, Chen HJ, Hsieh BC. Laminaria tent vs misoprostol for cervical priming before hysteroscopy: Randomized study. Journal of Minimally Invasive Gynecology 2009;16:708‐12. - PubMed
Oppegaard 2008 a {published data only}
Oppegaard 2008 b {published data only}
Oppegaard 2010 {published data only}
Preen 2002 {published data only}
-
- Preen AE, Jeffrey L, Keeby MC, et al. Oral misoprostol prior to operative hysteroscopy does not enhance cervical dilation or improve operative time: A prospective randomized, double blind, placebo‐controlled trial. Fertility and Sterility 2002;78:S79.
Preutthipan 1999 {published data only}
-
- Preutthipan S, Herabutya Y. A randomized controlled trial of vaginal misoprostol for cervical priming before hysteroscopy. Obstetrics and Gynecology 1999;94:427‐30. - PubMed
Preutthipan 2000 {published data only}
-
- Preutthipan S, Herabutya Y. Vaginal misoprostol for cervical priming before operative hysteroscopy: a randomized controlled trial. Obstetrics and Gynecology 2000;96:890‐4. - PubMed
Preutthipan 2006 {published data only}
-
- Preutthipan S, Herabutya Y. A randomized comparison of vaginal misoprostol and dinoprostone for cervical priming in nulliparous women before operative hysteroscopy. Fertility and Sterility 2006;86:990‐4. - PubMed
Song 2014 {published data only}
-
- Song T, Kim MK, Kim ML, Jung YW, Yoon BS, Seong SJ. Effectiveness of different routes of misoprostol administration before operative hysteroscopy: a randomized, controlled trial.. Fertil Steril. 2014;102:519‐24.. - PubMed
Thomas 2002 {published data only}
-
- Thomas JA, Leyland N, Durand N, Windrim RC. The use of oral misoprostol as a cervical ripening agent in operative hysteroscopy: a double‐blind, placebo‐controlled trial. American Journal of Obstetrics and Gynecology 2002;186:876‐9.i. - PubMed
Uckuyu 2008 {published data only}
-
- Uckuyu A, Ozcimen EE, Sevinc FC, Zeyneloglu HB. Efficacy of vaginal misoprostol before hysteroscopy for cervical priming in patients who have undergone cesarean section and no vaginal deliveries. Journal of Minimally Invasive Gynecology 2008;15:472‐5. - PubMed
Yu 2006 {published data only}
-
- YU D, Li T‐C, Xia E, Huang X. Aprospective randomized controlled trial comparing vaginal misoprostol and osmotic dilator in achieving cervical ripening before operative hysteroscopy. Gynecological Surgery 2006;3:186‐9.
References to studies excluded from this review
Esin 2013 {unpublished data only}
-
- Esin S, Baser E, Okuyan E, Kucukozkan T. Comparison of sublingual misoporstol versus lidocaine spray for pain relief in office hysteroscopy: a randomized, double blind, placebo‐controlled trial. Human Reproduction. 2013; Vol. 28(Supplement 1):i360‐66 P‐597. - PubMed
Fung 2002 {published data only}
-
- Fung TM, Lam MH, Wong SF, Ho LC. A randomized placebo‐controlled trial of vaginal misoprostol for cervical priming before hysteroscopy in post menopausal women. BJOG 2002;109:561‐5. - PubMed
Hald 1988 {published data only}
-
- Hald F, Kristoffersen SE, Gregersen E. Prostaglandin vaginal suppositories in non pregnant women requiring cervical dilatation prior to hysteroscopy. Acta Obstetricia et Gynecologica Scandinavica 1988;67:219‐22. - PubMed
Hassa 2013 {published data only}
-
- Hassa H, Aydin Y, Oge T, Cicek K. Effectiveness of vaginal misoprostol and rectal nonsteroida anti‐inflammatory drug in vaginoscopic diagnostic outpatien hysteroscopy in primarily infertile women: double‐blind, randomized, controlled trial. JMIG 2013;20(6):880‐5. - PubMed
Moiety 2012 {published data only}
-
- Moiety FMS, Azzam A. Prostaglandins prior to hysteroscopy. Gynecological Surgery 2012;9:169‐73.
Ngai 1997 {published data only}
-
- Ngai SW, Chan YM, Liu KL, Ho PC. Oral misoprostol for cervical priming in non‐pregnant women. Human Reproduction 1997;12:2373‐5. - PubMed
Ngai 2001 {published data only}
-
- Ngai SW, Chan YM, Ho PC. The use of misoprostol prior to hysteroscopy in post menopausal women. Human Reproduction 2001;16:1486‐8. - PubMed
Sofoudis 2012 {unpublished data only}
-
- Sofoudis C, Bakas P, Creatsa M, Kalampokas M, Grigoriou V, Vlahos N. A doble randomized cntrolled trial of oral misoprostol before diagnostic hysteroscopy. Gynecol Surg. 2012; Vol. 9(Suppl 1):S1‐S137.
Tanha 2013 {published data only}
-
- Tanha FD, Salimi S, Ghajarzadeh M. Sublingual versus vaginal misoprostol for cervical ripening before hysteroscopy: a randomized clinical trial. General Gynecology 2013;287:937‐40. - PubMed
References to studies awaiting assessment
Chencheewachat 2012 {unpublished data only}
-
- Chencheewachat C, Lertvikool S, Sukprasert M, Chanrachakul B. The efficacy of nitric oxide donor on cervical ripening prior to operative hysteroscopy: a randomized, double blinded, controlled trial. Reproductive Sciences. 2012; Vol. 19, No 3(Supplement).
Additional references
Agostini A 2002a
-
- Agostini A, Cravello L, Bretelle F, Shojai R, Roger V, Blanc B. Risk of uterine perforation during hysteroscopic surgery. The Journal of the American Association of Gynecologic Laparoscopists 2002a;9:264‐7. - PubMed
Bradley 2002
-
- Bradley LD. Complications in hysteroscopy: prevention, treatment and legal risk. Current Opinion in Obstetrics & Gynecology 2002;14:409‐15. - PubMed
Cooper 1996
-
- Cooper KG, Pinion SB, Bhattacharya S, Parkin DE. The effects of the gonadotrophin releasing hormone analogue (goserelin) and prostaglandin E1 (misoprostol) on cervical resistance prior to transcervical resection of the endometrium. British Journal Obstetrics and Gynaecology 1996;103:375‐8. - PubMed
Girgoriadis 2012
-
- Grigoriadis C, Papaconstantinou E, Mellou A, Hassiakos D, Liapis A, Kondi‐Pafiti A. Clinicopathological changes of uterine leiomyomas after GnRH agonist therapy. Clinical and Experimental Obstetrics & Gynecology 2012;39:191‐4. - PubMed
Gkrozou F 2011
-
- Gkrozou F1, Koliopoulos G, Vrekoussis T, Valasoulis G, Lavasidis L, Navrozoglou I, Paschopoulos M. A systematic review and meta‐analysis of randomized studies comparing misoprostol versus placebo for cervical ripening prior to hysteroscopy. Eur J Obstet Gynecol Reprod Biol. 2011;158:17‐23. - PubMed
Higgins 2003
Higgins 2011
-
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Loffer 1989
-
- Loffer. Hysteroscopy with selective endometrial sampling compared with D&C for abnormal uterine bleeding: the value of a negative hysteroscopic view. Obstetrics and Gynecology 1989;73:16‐20. - PubMed
Nagi 1997
-
- Ngai SW, Chan YM, Liu KL, Ho PC. Oral misoprostol for cervical priming in non‐pregnant women. Human Reproduction 1997;12:2373‐5. - PubMed
Ostrzenski 1994
-
- Ostrzenski A. Resectoscopic cervical trauma minimized by inserting Laminaria digitata preoperatively. International Journal of Fertility and Menopausal Studies 1994;39:111‐3. - PubMed
Overton 1997
-
- Overton C, Hargreaves J, Maresh M. A national survey of the complications of endometrial destruction for menstrual disorders: the MISTLETOE study. British Journal of Obstetrics and Gynaecology 1997;104:135‐9. - PubMed
Polyzos 2012
-
- Polyzos NP, Zavos A, Valachis A, et al. Misoprostol prior to hysteroscopy in premenopausal and post‐menopausal women. A systematic review and meta‐analysis. Human Reproduction Update 2012;18:393‐404. - PubMed
Rabe 1985
-
- Rabe T, Willinger G, Kiesel L, Runnebaum B, Kubili F, Mosche R, et al. Study of 16,16'‐dimethyl‐trans‐delta 2 prostaglandin E1 methyl ester vaginal suppository for cervical dilatation in premenopausal and postmenopausal women. Advances in Contraception 1985;1:91‐102. - PubMed
Sowter 2002
-
- Sowter MC, Lethaby A, Singla AA. Pre‐operative endometrial thinning agents before endometrial destruction for heavy menstrual bleeding. Cochrane Database of Systematic Reviews 2002;3:CD001124. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

