[(64) Cu]-labelled trastuzumab: optimisation of labelling by DOTA and NODAGA conjugation and initial evaluation in mice
- PMID: 25906708
- PMCID: PMC5029596
- DOI: 10.1002/jlcr.3287
[(64) Cu]-labelled trastuzumab: optimisation of labelling by DOTA and NODAGA conjugation and initial evaluation in mice
Abstract
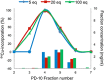
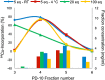
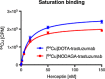
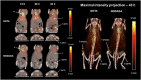
The human epidermal growth factor receptor-2 (HER2) is overexpressed in 20-30% of all breast cancer cases, leading to increased cell proliferation, growth and migration. The monoclonal antibody, trastuzumab, binds to HER2 and is used for treatment of HER2-positive breast cancer. Trastuzumab has previously been labelled with copper-64 by conjugation of a 1,4,7,10-tetraazacyclododecane-1,4,7,10-tetraacetic acid (DOTA) chelator. The aim of this study was to optimise the (64) Cu-labelling of DOTA-trastuzumab and as the first to produce and compare with its 1,4,7-triazacyclononane, 1-glutaric acid-5,7 acetic acid (NODAGA) analogue in a preliminary HER2 tumour mouse model. The chelators were conjugated to trastuzumab using the activated esters DOTA mono-N-hydroxysuccinimide (NHS) and NODAGA-NHS. (64) Cu-labelling of DOTA-trastuzumab was studied by varying the amount of DOTA-trastuzumab used, reaction temperature and time. Full (64) Cu incorporation could be achieved using a minimum of 10-µg DOTA-trastuzumab, but the fastest labelling was obtained after 15 min at room temperature using 25 µg of DOTA-trastuzumab. In comparison, 80% incorporation was achieved for (64) Cu-labelling of NODAGA-trastuzumab. Both [(64) Cu]DOTA-trastuzumab and [(64) Cu]NODAGA-trastuzumab were produced after purification with radiochemical purities of >97%. The tracers were injected into mice with HER2 expressing tumours. The mice were imaged by positron emission tomography and showed high tumour uptake of 3-9% ID/g for both tracers.
Keywords: DOTA; NODAGA; PET; trastuzumab.
© 2015 The Authors Journal of Labelled Compounds and Radiopharmaceuticals published by John Wiley & Sons Ltd.
Figures


Similar articles
-
Comparison of DOTA and NODAGA as chelators for (64)Cu-labeled immunoconjugates.Nucl Med Biol. 2015 Feb;42(2):177-83. doi: 10.1016/j.nucmedbio.2014.09.009. Epub 2014 Oct 5. Nucl Med Biol. 2015. PMID: 25457653
-
Comparative evaluation of synthetic anti-HER2 Affibody molecules site-specifically labelled with 111In using N-terminal DOTA, NOTA and NODAGA chelators in mice bearing prostate cancer xenografts.Eur J Nucl Med Mol Imaging. 2012 Mar;39(3):481-92. doi: 10.1007/s00259-011-1992-9. Epub 2011 Nov 30. Eur J Nucl Med Mol Imaging. 2012. PMID: 22322933
-
Imaging and biodistribution of Her2/neu expression in non-small cell lung cancer xenografts with Cu-labeled trastuzumab PET.Cancer Sci. 2010 Apr;101(4):1045-50. doi: 10.1111/j.1349-7006.2010.01480.x. Epub 2009 Dec 22. Cancer Sci. 2010. PMID: 20219072 Free PMC article.
-
Exploring utilities of [ 64 Cu]Cu-DOTA-trastuzumab immunoPET in breast cancer: a systematic review and meta-analysis.Nucl Med Commun. 2025 Apr 1;46(4):277-284. doi: 10.1097/MNM.0000000000001949. Epub 2025 Jan 21. Nucl Med Commun. 2025. PMID: 39834168
-
64Cu-Labeled Oxo-DO3A-conjugated trastuzumab and PCTA-conjugated trastuzumab.2012 Jul 24 [updated 2012 Aug 23]. In: Molecular Imaging and Contrast Agent Database (MICAD) [Internet]. Bethesda (MD): National Center for Biotechnology Information (US); 2004–2013. 2012 Jul 24 [updated 2012 Aug 23]. In: Molecular Imaging and Contrast Agent Database (MICAD) [Internet]. Bethesda (MD): National Center for Biotechnology Information (US); 2004–2013. PMID: 22934320 Free Books & Documents. Review.
Cited by
-
A Kinetically Controlled Bioconjugation Method for the Synthesis of Radioimmunoconjugates and the Development of a Domain Mapping MS-Workflow for Its Characterization.Bioconjug Chem. 2024 Mar 20;35(3):324-332. doi: 10.1021/acs.bioconjchem.3c00519. Epub 2024 Feb 17. Bioconjug Chem. 2024. PMID: 38366964 Free PMC article.
-
Comparison between Fractionated Dose and Single Dose of Cu-64 Trastuzumab Therapy in the NCI-N87 Gastric Cancer Mouse Model.Int J Mol Sci. 2019 Sep 23;20(19):4708. doi: 10.3390/ijms20194708. Int J Mol Sci. 2019. PMID: 31547586 Free PMC article.
-
Radiolabelled Peptides for Positron Emission Tomography and Endoradiotherapy in Oncology.Pharmaceuticals (Basel). 2020 Jan 30;13(2):22. doi: 10.3390/ph13020022. Pharmaceuticals (Basel). 2020. PMID: 32019275 Free PMC article. Review.
-
State of the Art in Radiolabeling of Antibodies with Common and Uncommon Radiometals for Preclinical and Clinical Immuno-PET.Bioconjug Chem. 2021 Jul 21;32(7):1315-1330. doi: 10.1021/acs.bioconjchem.1c00136. Epub 2021 May 11. Bioconjug Chem. 2021. PMID: 33974403 Free PMC article.
-
Radioimmunotheranostic Pair Based on the Anti-HER2 Monoclonal Antibody: Influence of Chelating Agents and Radionuclides on Biological Properties.Pharmaceutics. 2021 Jun 27;13(7):971. doi: 10.3390/pharmaceutics13070971. Pharmaceutics. 2021. PMID: 34198999 Free PMC article.
References
-
- Al‐Ejeh F., Darby J. M., Thierry B., Brown M. P., Nucl. Med. Biol. 2009, 36, 395. - PubMed
-
- Waldmann T. A., Science 1991, 252, 1657. - PubMed
-
- Price E. W., Orvig C., Chem. Soc. Rev. 2014, 43, 260. - PubMed
-
- Lewis M. R., Kao J. Y., Anderson A. L., Shively J. E., Raubitschek A., Bioconjugate Chem. 2001, 12, 320. - PubMed
-
- Wang W., Wang E. Q., Balthasar J. P., Clin. Pharmacol. Ther. 2008, 84, 548. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

