1,25(OH)2D3 downregulates the Toll-like receptor 4-mediated inflammatory pathway and ameliorates liver injury in diabetic rats
- PMID: 25906757
- PMCID: PMC4768236
- DOI: 10.1007/s40618-015-0287-6
1,25(OH)2D3 downregulates the Toll-like receptor 4-mediated inflammatory pathway and ameliorates liver injury in diabetic rats
Abstract
Background: Fatty acid deposition in the liver can activate a number of pro-inflammatory signaling pathways such as the Toll-like receptor 4 (TLR4) pathway, which may be important in the pathogenesis of nonalcoholic steatohepatitis. 1,25(OH)2D3 downregulates the expression of TLR4 and may represent a novel treatment strategy for reducing hepatocyte injury. Therefore, in this study, we investigated the protective effects of 1,25(OH)2D3 on diabetic liver injury in vivo.
Methods: Streptozotocin (STZ)-induced diabetic rats were randomly divided into five groups and treated with low-dose 1,25(OH)2D3 (0.025 μg/kg/day), medium-dose 1,25(OH)2D3 (0.15 μg/kg/day), high-dose 1,25(OH)2D3 (0.3 μg/kg/day), insulin (protamine zinc insulin 16 U/kg/day, subcutaneous injection), or no intervention (the control group). Sixteen weeks later, the rats were killed, and blood samples were obtained to test lipid profiles and hepatic function. The infiltration of inflammatory cells, the level of fibrosis, and the expression levels of TLR4, nuclear factor-kappa B (NF-κB), and tumor necrosis factor-α (TNF-α) in the liver were analyzed. The hepatocytes were treated with vehicle control, LPS (100 ng), high fat [DMEM + FFA (0.1 mM: palmitic acid, oleic acid, 1:2)], LPS + high fat, vehicle + 1,25(OH)2D3 (10(-7) M), LPS + 1,25(OH)2D3, high fat + 1,25(OH)2D3, or LPS + high fat + 1,25(OH)2D3. RNA and protein were extracted to detect the expression of TLR4 and downstream inflammatory factors such as NF-ΚB, TNF-α, and IL-6. Groups of data were compared by single factor variance analysis.
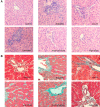
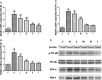
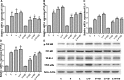
Results: High-dose 1,25(OH)2D3 administration for 16 weeks downregulated the expression of TLR4, NF-κB, and TNF-α in the liver tissue of diabetic rats and attenuated hepatic inflammation and fibrosis, as shown by immunohistochemical staining, hematoxylin and eosin staining, Masson's trichrome staining, reverse transcription polymerase chain reaction (RT-PCR), and western blotting. In vitro, hepatocytes treated with high fat or LPS exhibited significantly increased expression of TLR4, NF-κB, and downstream inflammatory factors (P < 0.05). Intervention with 1,25(OH)2D3 decreased the expression of TLR4, NF-κB, and inflammatory factors (P < 0.05).
Conclusions: 1,25(OH)2D3 exhibited protective effects against diabetes-related liver injury, possibly through downregulation of components of the TLR4 signaling pathway.
Keywords: 1,25(OH)2D3; Diabetes; Hepatocytes; Inflammation; Liver injury; Toll-like receptor 4.
Figures

References
-
- World Health Organization . Definition, diagnosis and classification of diabetes mellitus and its complications: report of a WHO Consultation. Part 1. Diagnosis and classification of diabetes mellitus. Geneva: World Health Organization; 1999.
-
- Wiedermann CJ, Kiechl S, Dunzendorfer S, Schratzberger P, Egger G, Oberhollenzer F, et al. Association of endotoxemia with carotid atherosclerosis and cardiovascular disease: prospective results from the Bruneck Study. J Am Coll Cardiol. 1999;34(7):1975–1981. doi: 10.1016/S0735-1097(99)00448-9. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

