Identification and functional characterization of the CYP51 gene from the yeast Xanthophyllomyces dendrorhous that is involved in ergosterol biosynthesis
- PMID: 25906980
- PMCID: PMC4415319
- DOI: 10.1186/s12866-015-0428-2
Identification and functional characterization of the CYP51 gene from the yeast Xanthophyllomyces dendrorhous that is involved in ergosterol biosynthesis
Abstract
Background: Xanthophyllomyces dendrorhous is a basidiomycetous yeast that synthesizes astaxanthin, a carotenoid with great biotechnological impact. The ergosterol and carotenoid synthetic pathways derive from the mevalonate pathway and involve cytochrome P450 enzymes. Among these enzymes, the CYP51 family, which is involved in ergosterol biosynthesis, is one of the most remarkable that has C14-demethylase activity.
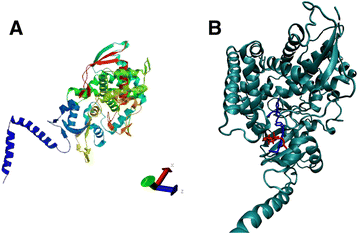
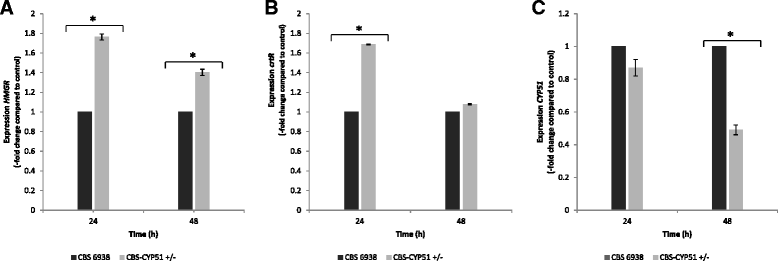
Results: In this study, the CYP51 gene from X. dendrorhous was isolated and its function was analyzed. The gene is composed of ten exons and encodes a predicted 550 amino acid polypeptide that exhibits conserved cytochrome P450 structural characteristics and shares significant identity with the sterol C14-demethylase from other fungi. The functionality of this gene was confirmed by heterologous complementation in S. cerevisiae. Furthermore, a CYP51 gene mutation in X. dendrorhous reduced sterol production by approximately 40% and enhanced total carotenoid production by approximately 90% compared to the wild-type strain after 48 and 120 h of culture, respectively. Additionally, the CYP51 gene mutation in X. dendrorhous increased HMGR (hydroxy-methylglutaryl-CoA reductase, involved in the mevalonate pathway) and crtR (cytochrome P450 reductase) transcript levels, which could be associated with reduced ergosterol production.
Conclusions: These results suggest that the CYP51 gene identified in X. dendrorhous encodes a functional sterol C14-demethylase that is involved in ergosterol biosynthesis.
Figures

Similar articles
-
Molecular Characterization and Functional Analysis of Cytochrome b5 Reductase (CBR) Encoding Genes from the Carotenogenic Yeast Xanthophyllomyces dendrorhous.PLoS One. 2015 Oct 14;10(10):e0140424. doi: 10.1371/journal.pone.0140424. eCollection 2015. PLoS One. 2015. PMID: 26466337 Free PMC article.
-
Enhancement of carotenoid production by disrupting the C22-sterol desaturase gene (CYP61) in Xanthophyllomyces dendrorhous.BMC Microbiol. 2012 Oct 18;12:235. doi: 10.1186/1471-2180-12-235. BMC Microbiol. 2012. PMID: 23075035 Free PMC article.
-
Enhancement of astaxanthin production in Xanthophyllomyces dendrorhous by efficient method for the complete deletion of genes.Microb Cell Fact. 2016 Sep 13;15(1):155. doi: 10.1186/s12934-016-0556-x. Microb Cell Fact. 2016. PMID: 27624332 Free PMC article.
-
The SREBP (Sterol Regulatory Element-Binding Protein) pathway: a regulatory bridge between carotenogenesis and sterol biosynthesis in the carotenogenic yeast Xanthophyllomyces dendrorhous.Biol Res. 2021 Oct 26;54(1):34. doi: 10.1186/s40659-021-00359-x. Biol Res. 2021. PMID: 34702374 Free PMC article. Review.
-
Cytochrome P450 lanosterol 14α-demethylase (CYP51): insights from molecular genetic analysis of the ERG11 gene in Saccharomyces cerevisiae.J Steroid Biochem Mol Biol. 1992 Dec;43(8):1107-16. doi: 10.1016/0960-0760(92)90339-K. J Steroid Biochem Mol Biol. 1992. PMID: 22217856 Review.
Cited by
-
Comparative analyses and structural insights of the novel cytochrome P450 fusion protein family CYP5619 in Oomycetes.Sci Rep. 2018 Apr 26;8(1):6597. doi: 10.1038/s41598-018-25044-0. Sci Rep. 2018. PMID: 29700357 Free PMC article.
-
Ergosterol Alleviates Kidney Injury in Streptozotocin-Induced Diabetic Mice.Evid Based Complement Alternat Med. 2015;2015:691594. doi: 10.1155/2015/691594. Epub 2015 Nov 17. Evid Based Complement Alternat Med. 2015. PMID: 26664454 Free PMC article.
-
Functional characterization of thiolase-encoding genes from Xanthophyllomyces dendrorhous and their effects on carotenoid synthesis.BMC Microbiol. 2016 Nov 21;16(1):278. doi: 10.1186/s12866-016-0893-2. BMC Microbiol. 2016. PMID: 27871246 Free PMC article.
-
Molecular Characterization and Functional Analysis of Cytochrome b5 Reductase (CBR) Encoding Genes from the Carotenogenic Yeast Xanthophyllomyces dendrorhous.PLoS One. 2015 Oct 14;10(10):e0140424. doi: 10.1371/journal.pone.0140424. eCollection 2015. PLoS One. 2015. PMID: 26466337 Free PMC article.
-
Comparative Transcriptome Analysis of Gill Tissue in Response to Hypoxia in Silver Sillago (Sillago sihama).Animals (Basel). 2020 Apr 6;10(4):628. doi: 10.3390/ani10040628. Animals (Basel). 2020. PMID: 32268576 Free PMC article.
References
-
- The Global Market for Carotenoids. Report Code: FOD025D. [http://www.bccresearch.com/pressroom/fod/global-carotenoids-market-reach...]
-
- Schroeder WA, Johnson EA. Antioxidant role of carotenoids in Phaffia rhodozyma. J Gen Microbiol. 1993;139:907–12. doi: 10.1099/00221287-139-5-907. - DOI
-
- Schroeder WA, Johnson EA. Carotenoids protect Phaffia rhodozyma against singlet oxygen damage. J Ind Microbiol. 1995;14:502–7. doi: 10.1007/BF01573965. - DOI
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

