Fabrication and characterization of medical grade polyurethane composite catheters for near-infrared imaging
- PMID: 25907050
- PMCID: PMC4417621
- DOI: 10.1016/j.biomaterials.2015.03.020
Fabrication and characterization of medical grade polyurethane composite catheters for near-infrared imaging
Abstract
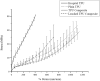
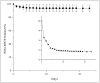
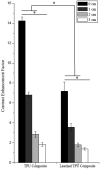
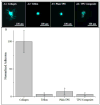
Peripherally inserted central catheters (PICCs) are hollow polymeric tubes that transport nutrients, blood and medications to neonates. To determine proper PICC placement, frequent X-ray imaging of neonates is performed. Because X-rays pose severe health risks to neonates, safer alternatives are needed. We hypothesize that near infrared (NIR) polymer composites can be fabricated into catheters by incorporating a fluorescent dye (IRDye 800CW) and visualized using NIR imaging. To fabricate catheters, polymer and dye are dry mixed and pressed, sectioned, and extruded to produce hollow tubes. We analyzed surface roughness, stiffness, dye retention, NIR contrast intensity, and biocompatibility. The extrusion process did not significantly alter the mechanical properties of the polymer composites. Over a period of 23 days, only 6.35 ± 5.08% dye leached out of catheters. The addition of 0.025 wt% dye resulted in a 14-fold contrast enhancement producing clear PICC images at 1 cm under a tissue equivalent. The addition of IRDye 800CW did not alter the biocompatibility of the polymer and did not increase adhesion of cells to the surface. We successfully demonstrated that catheters can be imaged without the use of harmful radiation and still maintain the same properties as the unaltered medical grade equivalent.
Keywords: Catheter; Cell adhesion; Fluorescence; Mechanical testing; Polyurethane.
Copyright © 2015 Elsevier Ltd. All rights reserved.
Figures




References
-
- McCay AS, Elliott EC, Walden M. PICC Placement in the Neonate. New England Journal of Medicine. 2014;370(11) - PubMed
-
- Vo JN, Hoffer FA, Shaw DW. Techniques in vascular and interventional radiology: pediatric central venous access. Techniques in vascular and interventional radiology. 2010;13(4):250–7. - PubMed
-
- Hogan MJ. Neonatal vascular catheters and their complications. Radiologic Clinics of North America. 1999;37(6):1109–25. - PubMed
-
- Sharpe EL. Repositioning techniques for malpositioned neonatal peripherally inserted central catheters. Advances in Neonatal Care. 2010;10(3):129–32. - PubMed
-
- Patricia Catudal J, Sharpe EL. The Wandering Ways of a PICC Line: Case Report of a Malpositioned Peripherally Inserted Central Catheter (PICC) and Correction. Journal of the Association for Vascular Access. 2011;16(4):218–20.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

