A Novel Peroxisome Proliferator-activated Receptor (PPAR)α Agonist and PPARγ Antagonist, Z-551, Ameliorates High-fat Diet-induced Obesity and Metabolic Disorders in Mice
- PMID: 25907553
- PMCID: PMC4505524
- DOI: 10.1074/jbc.M114.622191
A Novel Peroxisome Proliferator-activated Receptor (PPAR)α Agonist and PPARγ Antagonist, Z-551, Ameliorates High-fat Diet-induced Obesity and Metabolic Disorders in Mice
Abstract
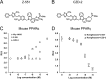
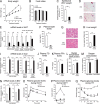
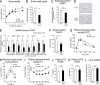
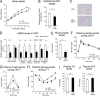
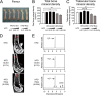
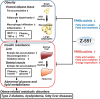
A novel peroxisome proliferator-activated receptor (PPAR) modulator, Z-551, having both PPARα agonistic and PPARγ antagonistic activities, has been developed for the treatment of obesity and obesity-related metabolic disorders. We examined the effects of Z-551 on obesity and the metabolic disorders in wild-type mice on the high-fat diet (HFD). In mice on the HFD, Z-551 significantly suppressed body weight gain and ameliorated insulin resistance and abnormal glucose and lipid metabolisms. Z-551 inhibited visceral fat mass gain and adipocyte hypertrophy, and reduced molecules involved in fatty acid uptake and synthesis, macrophage infiltration, and inflammation in adipose tissue. Z-551 increased molecules involved in fatty acid combustion, while reduced molecules associated with gluconeogenesis in the liver. Furthermore, Z-551 significantly reduced fasting plasma levels of glucose, triglyceride, free fatty acid, insulin, and leptin. To elucidate the significance of the PPAR combination, we examined the effects of Z-551 in PPARα-deficient mice and those of a synthetic PPARγ antagonist in wild-type mice on the HFD. Both drugs showed similar, but weaker effects on body weight, insulin resistance and specific events provoked in adipose tissue compared with those of Z-551 as described above, except for lack of effects on fasting plasma triglyceride and free fatty acid levels. These findings suggest that Z-551 ameliorates HFD-induced obesity, insulin resistance, and impairment of glucose and lipid metabolisms by PPARα agonistic and PPARγ antagonistic activities, and therefore, might be clinically useful for preventing or treating obesity and obesity-related metabolic disorders such as insulin resistance, type 2 diabetes, and dyslipidemia.
Keywords: adipose tissue; diabetes; dyslipidemia; insulin resistance; liver; metabolic disorders; obesity; peroxisome proliferator-activated receptor (PPAR).
© 2015 by The American Society for Biochemistry and Molecular Biology, Inc.
Figures



References
-
- Flegal K. M., Carroll M. D., Ogden C. L., Curtin L. R. (2010) Prevalence and trends in obesity among US adults, 1999–2008. JAMA 303, 235–241 - PubMed
-
- Kopelman P. G. (2000) Obesity as a medical problem. Nature 404, 635–643 - PubMed
-
- Ahima R. S. (2006) Adipose tissue as an endocrine organ. Obesity 14, 242S–249S - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

