The Us3 Protein of Herpes Simplex Virus 1 Inhibits T Cell Signaling by Confining Linker for Activation of T Cells (LAT) Activation via TRAF6 Protein
- PMID: 25907557
- PMCID: PMC4505477
- DOI: 10.1074/jbc.M115.646422
The Us3 Protein of Herpes Simplex Virus 1 Inhibits T Cell Signaling by Confining Linker for Activation of T Cells (LAT) Activation via TRAF6 Protein
Abstract
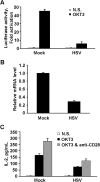
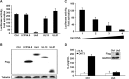
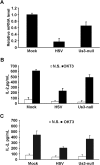
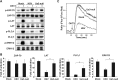
Herpes simplex virus 1 (HSV-1) is the most prevalent human virus and causes global morbidity because the virus is able to infect multiple cell types. Remarkably, HSV infection switches between lytic and latent cycles, where T cells play a critical role. However, the precise way of virus-host interactions is incompletely understood. Here we report that HSV-1 productively infected Jurkat T-cells and inhibited antigen-induced T cell receptor activation. We discovered that HSV-1-encoded Us3 protein interrupted TCR signaling and interleukin-2 production by inactivation of the linker for activation of T cells. This study unveils a mechanism by which HSV-1 intrudes into early events of TCR-mediated cell signaling and may provide novel insights into HSV infection, during which the virus escapes from host immune surveillance.
Keywords: HSV-1; LAT; TCR; TRAF6; Us3; interleukin; signaling; ubiquitination.
© 2015 by The American Society for Biochemistry and Molecular Biology, Inc.
Figures


Similar articles
-
The US3 Kinase of Herpes Simplex Virus Phosphorylates the RNA Sensor RIG-I To Suppress Innate Immunity.J Virol. 2022 Feb 23;96(4):e0151021. doi: 10.1128/JVI.01510-21. Epub 2021 Dec 22. J Virol. 2022. PMID: 34935440 Free PMC article.
-
Herpes simplex virus US3 tegument protein inhibits Toll-like receptor 2 signaling at or before TRAF6 ubiquitination.Virology. 2013 May 10;439(2):65-73. doi: 10.1016/j.virol.2013.01.026. Epub 2013 Mar 7. Virology. 2013. PMID: 23478027 Free PMC article.
-
Herpes simplex virus type 1 Us3 gene deletion influences toll-like receptor responses in cultured monocytic cells.Virol J. 2008 Nov 21;5:140. doi: 10.1186/1743-422X-5-140. Virol J. 2008. PMID: 19025601 Free PMC article.
-
[Molecular mechanism by which Us3 protein kinase regulates the pathogenicity of herpes simplex virus type-1].Uirusu. 2016;66(1):83-90. doi: 10.2222/jsv.66.83. Uirusu. 2016. PMID: 28484184 Review. Japanese.
-
Us3 Protein Kinase Encoded by HSV: The Precise Function and Mechanism on Viral Life Cycle.Adv Exp Med Biol. 2018;1045:45-62. doi: 10.1007/978-981-10-7230-7_3. Adv Exp Med Biol. 2018. PMID: 29896662 Review.
Cited by
-
Understanding the interplay between oHSV and the host immune system: Implications for therapeutic oncolytic virus development.Mol Ther. 2025 Apr 2;33(4):1327-1343. doi: 10.1016/j.ymthe.2024.12.054. Epub 2024 Dec 30. Mol Ther. 2025. PMID: 39741405 Review.
-
US6 Gene Deletion in Herpes Simplex Virus Type 2 Enhances Dendritic Cell Function and T Cell Activation.Front Immunol. 2017 Nov 10;8:1523. doi: 10.3389/fimmu.2017.01523. eCollection 2017. Front Immunol. 2017. PMID: 29176979 Free PMC article.
-
Characteristics of herpes simplex virus infection and pathogenesis suggest a strategy for vaccine development.Rev Med Virol. 2019 Jul;29(4):e2054. doi: 10.1002/rmv.2054. Epub 2019 Jun 13. Rev Med Virol. 2019. PMID: 31197909 Free PMC article. Review.
-
Oncolytic herpes simplex virus interactions with the host immune system.Curr Opin Virol. 2016 Dec;21:26-34. doi: 10.1016/j.coviro.2016.07.007. Epub 2016 Aug 3. Curr Opin Virol. 2016. PMID: 27497296 Free PMC article. Review.
-
Quantitative Trait Locus Based Virulence Determinant Mapping of the HSV-1 Genome in Murine Ocular Infection: Genes Involved in Viral Regulatory and Innate Immune Networks Contribute to Virulence.PLoS Pathog. 2016 Mar 10;12(3):e1005499. doi: 10.1371/journal.ppat.1005499. eCollection 2016 Mar. PLoS Pathog. 2016. PMID: 26962864 Free PMC article.
References
-
- Whitley R. J., Roizman B. (2001) Herpes simplex virus infections. Lancet 357, 1513–1518 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

