MicroRNA-26a/-26b-COX-2-MIP-2 Loop Regulates Allergic Inflammation and Allergic Inflammation-promoted Enhanced Tumorigenic and Metastatic Potential of Cancer Cells
- PMID: 25907560
- PMCID: PMC4447993
- DOI: 10.1074/jbc.M115.645580
MicroRNA-26a/-26b-COX-2-MIP-2 Loop Regulates Allergic Inflammation and Allergic Inflammation-promoted Enhanced Tumorigenic and Metastatic Potential of Cancer Cells
Abstract
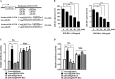
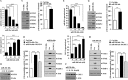
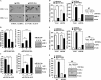
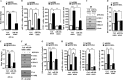
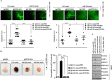
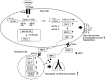
Cyclooxgenase-2 (COX-2) knock-out mouse experiments showed that COX-2 was necessary for in vivo allergic inflammation, such as passive cutaneous anaphylaxis, passive systemic anaphylaxis, and triphasic cutaneous allergic reaction. TargetScan analysis predicted COX-2 as a target of miR-26a and miR-26b. miR-26a/-26b decreased luciferase activity associated with COX-2-3'-UTR. miR-26a/-26b exerted negative effects on the features of in vitro and in vivo allergic inflammation by targeting COX-2. ChIP assays showed the binding of HDAC3 and SNAIL, but not COX-2, to the promoter sequences of miR-26a and miR-26b. Cytokine array analysis showed that the induction of chemokines, such as MIP-2, in the mouse passive systemic anaphylaxis model occurred in a COX-2-dependent manner. ChIP assays showed the binding of HDAC3 and COX-2 to the promoter sequences of MIP-2. In vitro and in vivo allergic inflammation was accompanied by the increased expression of MIP-2. miR-26a/-26b negatively regulated the expression of MIP-2. Allergic inflammation enhanced the tumorigenic and metastatic potential of cancer cells and induced positive feedback involving cancer cells and stromal cells, such as mast cells, macrophages, and endothelial cells. miR-26a mimic and miR-26b mimic negatively regulated the positive feedback between cancer cells and stromal cells and the positive feedback among stromal cells. miR-26a/-26b negatively regulated the enhanced tumorigenic potential by allergic inflammation. COX-2 was necessary for the enhanced metastatic potential of cancer cells by allergic inflammation. Taken together, our results indicate that the miR26a/-26b-COX-2-MIP-2 loop regulates allergic inflammation and the feedback relationship between allergic inflammation and the enhanced tumorigenic and metastatic potential.
Keywords: COX-2; MIP-2; allergic inflammation; allergy; angiogenesis; metastasis; miR-26; microRNA (miRNA); tumor microenvironment; tumorigenic potential.
© 2015 by The American Society for Biochemistry and Molecular Biology, Inc.
Figures











Similar articles
-
Transglutaminase II/microRNA-218/-181a loop regulates positive feedback relationship between allergic inflammation and tumor metastasis.J Biol Chem. 2014 Oct 24;289(43):29483-505. doi: 10.1074/jbc.M114.603480. Epub 2014 Sep 8. J Biol Chem. 2014. PMID: 25202021 Free PMC article.
-
MiR-135-5p-p62 Axis Regulates Autophagic Flux, Tumorigenic Potential, and Cellular Interactions Mediated by Extracellular Vesicles During Allergic Inflammation.Front Immunol. 2019 Apr 5;10:738. doi: 10.3389/fimmu.2019.00738. eCollection 2019. Front Immunol. 2019. PMID: 31024564 Free PMC article.
-
EGR3-HDAC6-IL-27 Axis Mediates Allergic Inflammation and Is Necessary for Tumorigenic Potential of Cancer Cells Enhanced by Allergic Inflammation-Promoted Cellular Interactions.Front Immunol. 2021 Jun 21;12:680441. doi: 10.3389/fimmu.2021.680441. eCollection 2021. Front Immunol. 2021. PMID: 34234781 Free PMC article.
-
Histone deacetylase-3 mediates positive feedback relationship between anaphylaxis and tumor metastasis.J Biol Chem. 2014 Apr 25;289(17):12126-12144. doi: 10.1074/jbc.M113.521245. Epub 2014 Mar 11. J Biol Chem. 2014. PMID: 24619412 Free PMC article.
-
MiR-375: A novel multifunctional regulator.Life Sci. 2021 Jun 15;275:119323. doi: 10.1016/j.lfs.2021.119323. Epub 2021 Mar 17. Life Sci. 2021. PMID: 33744323 Review.
Cited by
-
miR-26a Limits Muscle Wasting and Cardiac Fibrosis through Exosome-Mediated microRNA Transfer in Chronic Kidney Disease.Theranostics. 2019 Mar 7;9(7):1864-1877. doi: 10.7150/thno.29579. eCollection 2019. Theranostics. 2019. PMID: 31037144 Free PMC article.
-
Vitamin D stimulates miR-26b-5p to inhibit placental COX-2 expression in preeclampsia.Sci Rep. 2021 May 27;11(1):11168. doi: 10.1038/s41598-021-90605-9. Sci Rep. 2021. PMID: 34045549 Free PMC article.
-
Interplay between cyclooxygenase‑2 and microRNAs in cancer (Review).Mol Med Rep. 2021 May;23(5):347. doi: 10.3892/mmr.2021.11986. Epub 2021 Mar 24. Mol Med Rep. 2021. PMID: 33760116 Free PMC article.
-
Circulating Neutrophil MicroRNAs as Biomarkers for the Detection of Lung Cancer.Biomark Cancer. 2016 Jan 24;8:1-7. doi: 10.4137/BIC.S37333. eCollection 2016. Biomark Cancer. 2016. PMID: 26823654 Free PMC article.
-
The translational potential of miR-26 in atherosclerosis and development of agents for its target genes ACC1/2, COL1A1, CPT1A, FBP1, DGAT2, and SMAD7.Cardiovasc Diabetol. 2024 Jan 9;23(1):21. doi: 10.1186/s12933-024-02119-z. Cardiovasc Diabetol. 2024. PMID: 38195542 Free PMC article. Review.
References
-
- Jeon C. M., Shin I. S., Shin N. R., Hong J. M., Kwon O. K., Kim H. S., Oh S. R., Myung P. K., Ahn K. S. (2014) Siegesbeckia glabrescens attenuates allergic airway inflammation in LPS-stimulated RAW 264.7 cells and OVA induced asthma murine model. Int. Immunopharmacol. 22, 414–419 - PubMed
-
- Huang W. C., Chai C. Y., Chen W. C., Hou M. F., Wang Y. S., Chiu Y. C., Lu S. R., Chang W. C., Juo S. H., Wang J. Y., Chang W. C. (2011) Histamine regulates cyclooxygenase 2 gene activation through Orai1-mediated NFκB activation in lung cancer cells. Cell Calcium 50, 27–35 - PubMed
-
- Shiraishi Y., Asano K., Niimi K., Fukunaga K., Wakaki M., Kagyo J., Takihara T., Ueda S., Nakajima T., Oguma T., Suzuki Y., Shiomi T., Sayama K., Kagawa S., Ikeda E., Hirai H., Nagata K., Nakamura M., Miyasho T., Ishizaka A. (2008) Cyclooxygenase-2/prostaglandin D2/CRTH2 pathway mediates double-stranded RNA-induced enhancement of allergic airway inflammation. J. Immunol. 180, 541–549 - PubMed
-
- Passos G. F., Medeiros R., Marcon R., Nascimento A. F., Calixto J. B., Pianowski L. F. (2013) The role of PKC/ERK1/2 signaling in the anti-inflammatory effect of tetracyclic triterpene euphol on TPA-induced skin inflammation in mice. Eur. J. Pharmacol. 698, 413–420 - PubMed
-
- Morin C., Fortin S., Cantin A. M., Rousseau É. (2013) MAG-EPA resolves lung inflammation in an allergic model of asthma. Clin. Exp. Allergy 43, 1071–1082 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

