Recurrent gain of function mutation in calcium channel CACNA1H causes early-onset hypertension with primary aldosteronism
- PMID: 25907736
- PMCID: PMC4408447
- DOI: 10.7554/eLife.06315
Recurrent gain of function mutation in calcium channel CACNA1H causes early-onset hypertension with primary aldosteronism
Abstract
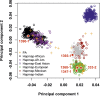
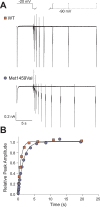
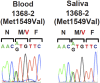
Many Mendelian traits are likely unrecognized owing to absence of traditional segregation patterns in families due to causation by de novo mutations, incomplete penetrance, and/or variable expressivity. Genome-level sequencing can overcome these complications. Extreme childhood phenotypes are promising candidates for new Mendelian traits. One example is early onset hypertension, a rare form of a global cause of morbidity and mortality. We performed exome sequencing of 40 unrelated subjects with hypertension due to primary aldosteronism by age 10. Five subjects (12.5%) shared the identical, previously unidentified, heterozygous CACNA1H(M1549V) mutation. Two mutations were demonstrated to be de novo events, and all mutations occurred independently. CACNA1H encodes a voltage-gated calcium channel (CaV3.2) expressed in adrenal glomerulosa. CACNA1H(M1549V) showed drastically impaired channel inactivation and activation at more hyperpolarized potentials, producing increased intracellular Ca(2+), the signal for aldosterone production. This mutation explains disease pathogenesis and provides new insight into mechanisms mediating aldosterone production and hypertension.
Keywords: CaV3.2; adrenal gland; chromosomes; de novo mutation; exome sequencing; genes; human; human biology; incomplete penetrance; medicine; voltage-gated calcium channel.
Conflict of interest statement
The authors declare that no competing interests exist.
Figures






References
-
- Azizan EA, Poulsen H, Tuluc P, Zhou J, Clausen MV, Lieb A, Maniero C, Garg S, Bochukova EG, Zhao W, Shaikh LH, Brighton CA, Teo AE, Davenport AP, Dekkers T, Tops B, Küsters B, Ceral J, Yeo GS, Neogi SG, McFarlane I, Rosenfeld N, Marass F, Hadfield J, Margas W, Chaggar K, Solar M, Deinum J, Dolphin AC, Farooqi IS, Striessnig J, Nissen P, Brown MJ. Somatic mutations in ATP1A1 and CACNA1D underlie a common subtype of adrenal hypertension. Nature Genetics. 2013;45:1055–1060. doi: 10.1038/ng.2716. - DOI - PubMed
-
- Beuschlein F, Boulkroun S, Osswald A, Wieland T, Nielsen HN, Lichtenauer UD, Penton D, Schack VR, Amar L, Fischer E, Walther A, Tauber P, Schwarzmayr T, Diener S, Graf E, Allolio B, Samson-Couterie B, Benecke A, Quinkler M, Fallo F, Plouin PF, Mantero F, Meitinger T, Mulatero P, Jeunemaitre X, Warth R, Vilsen B, Zennaro MC, Strom TM, Reincke M. Somatic mutations in ATP1A1 and ATP2B3 lead to aldosterone-producing adenomas and secondary hypertension. Nature Genetics. 2013;45:440–444. doi: 10.1038/ng.2550. - DOI - PubMed
-
- Bock G, Gebhart M, Scharinger A, Jangsangthong W, Busquet P, Poggiani C, Sartori S, Mangoni ME, Sinnegger-Brauns MJ, Herzig S, Striessnig J, Koschak A. Functional properties of a newly identified C-terminal splice variant of Cav1.3 L-type Ca2+ channels. The Journal of Biological Chemistry. 2011;286:42736–42748. doi: 10.1074/jbc.M111.269951. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

