Adipose cells promote resistance of breast cancer cells to trastuzumab-mediated antibody-dependent cellular cytotoxicity
- PMID: 25908175
- PMCID: PMC4482271
- DOI: 10.1186/s13058-015-0569-0
Adipose cells promote resistance of breast cancer cells to trastuzumab-mediated antibody-dependent cellular cytotoxicity
Abstract
Introduction: Trastuzumab has been used in the treatment of human epidermal growth factor receptor 2 (HER2)-expressing breast cancer, but its efficacy is limited by de novo or acquired resistance. Although many mechanisms have been proposed to explain resistance to trastuzumab, little is known concerning the role of the tumor microenvironment. Given the importance of antibody-dependent cellular cytotoxicity (ADCC) in the antitumor effect of trastuzumab and the abundance of adipose tissue in the breast, we investigated the impact of adipocytes on ADCC.
Methods: We set up a coculture system to study the effect of adipocytes on ADCC in vitro. The results were validated in vivo in a mouse xenograft model.
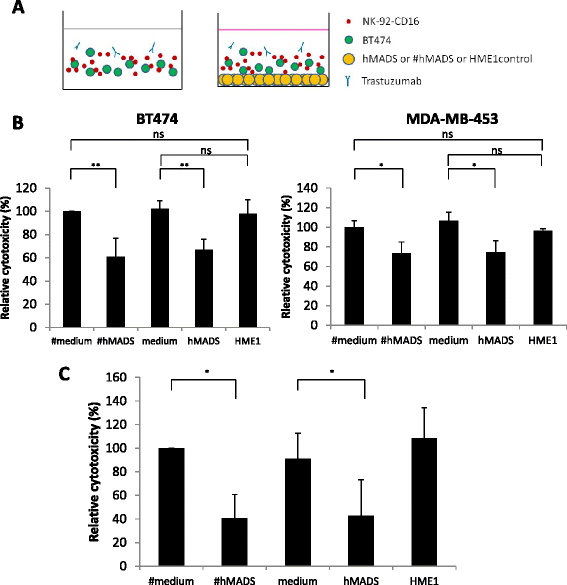
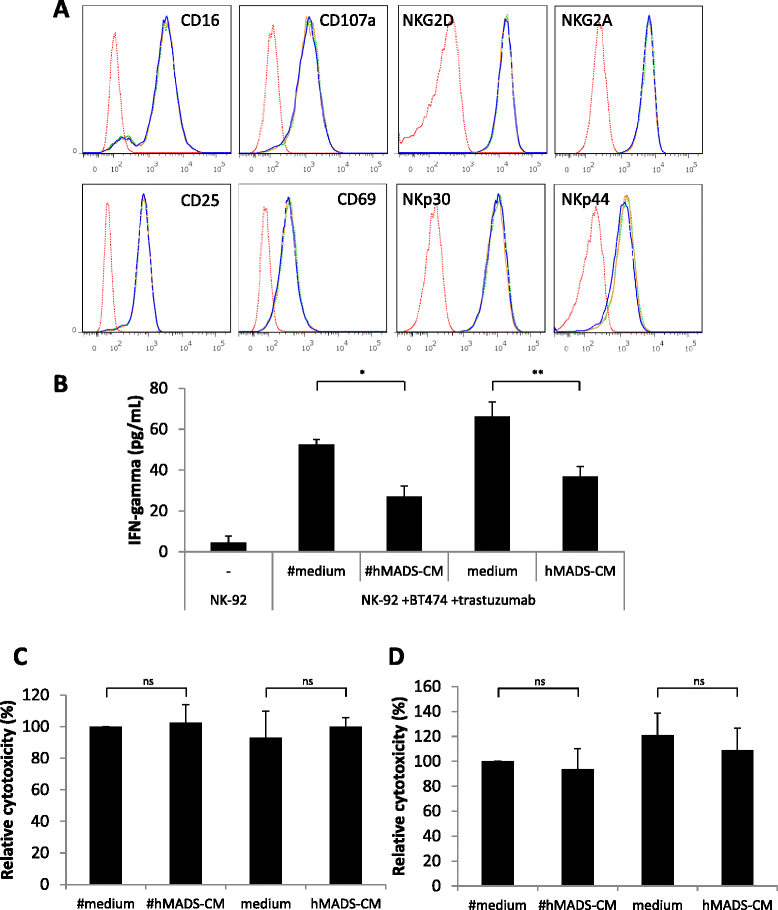
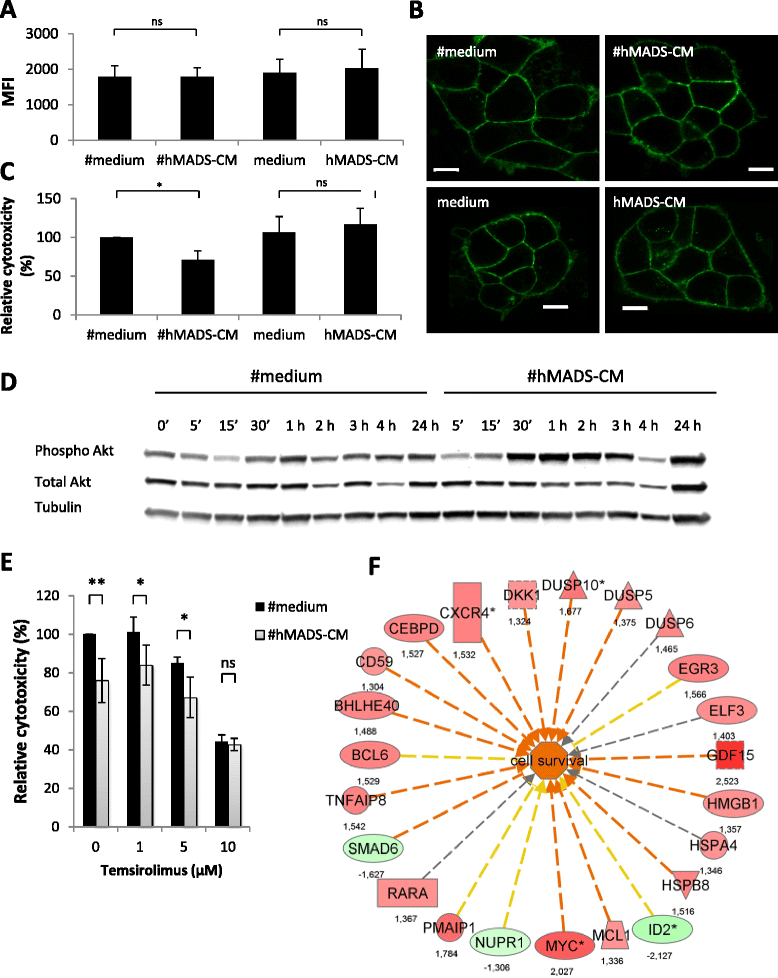
Results: We found that adipocytes, as well as preadipocytes, inhibited trastuzumab-mediated ADCC in HER2-expressing breast cancer cells via the secretion of soluble factors. The inhibition of ADCC was not due to titration or degradation of the antibody. We found that adipose cells decreased the secretion of interferon-γ by natural killer cells, but did not alter natural killer cells' cytotoxicity. Preincubation of breast cancer cells with the conditioned medium derived from adipocytes reduced the sensitivity of cancer cells to ADCC. Using a transcriptomic approach, we found that cancer cells undergo major modifications when exposed to adipocyte-conditioned medium. Importantly, breast tumors grafted next to lipomas displayed resistance to trastuzumab in mouse xenograft models.
Conclusions: Collectively, our findings underline the importance of adipose tissue in the resistance to trastuzumab and suggest that approaches targeting the adipocyte-cancer cell crosstalk may help sensitize cancer cells to trastuzumab-based therapy.
Figures



References
-
- Musolino A, Naldi N, Bortesi B, Pezzuolo D, Capelletti M, Missale G, et al. Immunoglobulin G fragment C receptor polymorphisms and clinical efficacy of trastuzumab-based therapy in patients with HER-2/neu–positive metastatic breast cancer. J Clin Oncol. 2008;26:1789–96. doi: 10.1200/JCO.2007.14.8957. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

