Pro-Permeability Factors After Dexamethasone Implant in Retinal Vein Occlusion; the Ozurdex for Retinal Vein Occlusion (ORVO) Study
- PMID: 25908486
- PMCID: PMC6600806
- DOI: 10.1016/j.ajo.2015.04.025
Pro-Permeability Factors After Dexamethasone Implant in Retinal Vein Occlusion; the Ozurdex for Retinal Vein Occlusion (ORVO) Study
Abstract
Purpose: To correlate aqueous vasoactive protein changes with macular edema after dexamethasone implant in retinal vein occlusion (RVO).
Design: Prospective, interventional case series.
Methods: Twenty-three central RVO (CRVO) and 17 branch RVO (BRVO) subjects with edema despite prior anti-vascular endothelial growth factor (VEGF) treatment had aqueous taps at baseline and 4 and 16 weeks after dexamethasone implant. Best-corrected visual acuity (BCVA) and center subfield thickness were measured every 4 weeks. Aqueous vasoactive protein levels were measured by protein array or enzyme-linked immunosorbent assay.
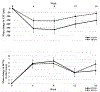
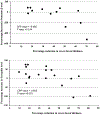
Results: Thirty-two vasoactive proteins were detected in aqueous in untreated eyes with macular edema due to RVO. Reduction in excess foveal thickness after dexamethasone implant correlated with reduction in persephin and pentraxin 3 (Pearson correlation coefficients = 0.682 and 0.638, P = .014 and P = .003). Other protein changes differed among RVO patients as edema decreased, but ≥50% of patients showed reductions in hepatocyte growth factor, endocrine gland VEGF, insulin-like growth factor binding proteins, or endostatin by ≥30%. Enzyme-linked immunosorbent assay in 18 eyes (12 CRVO, 6 BRVO) showed baseline levels of hepatocyte growth factor and VEGF of 168.2 ± 20.1 pg/mL and 78.7 ± 10.0 pg/mL, and each was reduced in 12 eyes after dexamethasone implant.
Conclusions: Dexamethasone implants reduce several pro-permeability proteins providing a multitargeted approach in RVO. No single protein in addition to VEGF can be implicated as a contributor in all patients. Candidates for contribution to chronic edema in subgroups of patients that deserve further study include persephin, hepatocyte growth factor, and endocrine gland VEGF.
Copyright © 2015 Elsevier Inc. All rights reserved.
Figures
Comment in
-
Pro-permeability Factors After Dexamethasone Implant in Retinal Vein Occlusion; The Ozurdex for Retinal Vein Occlusion (ORVO) Study.Am J Ophthalmol. 2016 Jan;161:215-6. doi: 10.1016/j.ajo.2015.09.035. Epub 2015 Oct 23. Am J Ophthalmol. 2016. PMID: 26477695 No abstract available.
-
Reply.Am J Ophthalmol. 2016 Jan;161:216-7. doi: 10.1016/j.ajo.2015.09.034. Epub 2015 Oct 23. Am J Ophthalmol. 2016. PMID: 26482397 No abstract available.
References
-
- Campochiaro PA, Hafiz G, Shah SM, et al. Ranibizumab for macular edema due to retinal vein occlusions; implication of VEGF as a critical stimulator. Molec Ther 2008;16(4):791–799. - PubMed
-
- Campochiaro PA, Hafiz G, Channa R, et al. Antagonism of vascular endothelial growth factor for macular edema caused by retinal vein occlusion: two-year outcomes. Ophthalmology 2010;117(12):2387–2394. - PubMed
-
- Brown DM, Campochiaro PA, Singh RP, et al. Efficacy and safety of ranibizumab in the treatment of macular edema secondary to central retinal vein occlusion:6-month results of the phase III CRUISE study. Ophthalmology 2010;117(6):1124–1133. - PubMed
-
- Campochiaro PA, Brown DM, Awh CC, et al. Sustained benefits from ranibizumab for macular edema following central retinal vein occlusion: 12-month outcomes of a phase III study. Ophthalmology 2011;118(10):2041–2049. - PubMed
-
- Brown DM, Campochiaro PA, Bhisitkul RB, et al. Sustained benefits from ranibizumab for macular edema following branch retinal vein occlusion: 12-month outcomes of a phase III study. Ophthalmology 2011. ;118(8):1594–1602. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

