Challenges in initiating and conducting personalized cancer therapy trials: perspectives from WINTHER, a Worldwide Innovative Network (WIN) Consortium trial
- PMID: 25908602
- PMCID: PMC5006164
- DOI: 10.1093/annonc/mdv191
Challenges in initiating and conducting personalized cancer therapy trials: perspectives from WINTHER, a Worldwide Innovative Network (WIN) Consortium trial
Abstract
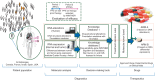
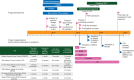
Advances in 'omics' technology and targeted therapeutic molecules are together driving the incorporation of molecular-based diagnostics into the care of patients with cancer. There is an urgent need to assess the efficacy of therapy determined by molecular matching of patients with particular targeted therapies. WINTHER is a clinical trial that uses cutting edge genomic and transcriptomic assays to guide treatment decisions. Through the lens of this ambitious multinational trial (five countries, six sites) coordinated by the Worldwide Innovative Networking Consortium for personalized cancer therapy, we discovered key challenges in initiation and conduct of a prospective, omically driven study. To date, the time from study concept to activation has varied between 19 months at Gustave Roussy Cancer Campus in France to 30 months at the Segal Cancer Center, McGill University (Canada). It took 3+ years to be able to activate US sites due to national regulatory hurdles. Access to medications proposed by the molecular analysis remains a major challenge, since their availability through active clinical trials is highly variable over time within sites and across the network. Rules regarding the off-label use of drugs, or drugs not yet approved at all in some countries, pose a further challenge, and many biopharmaceutical companies lack a simple internal mechanism to supply the drugs even if they wish to do so. These various obstacles should be addressed to test and then implement precision medicine in cancer.
Keywords: biomarker; clinical trials; personalized cancer therapy.
© The Author 2015. Published by Oxford University Press on behalf of the European Society for Medical Oncology. All rights reserved. For permissions, please email: journals.permissions@oup.com.
Figures
References
-
- Karapetis CS, Khambata-Ford S, Jonker DJ et al. . K-ras mutations and benefit from cetuximab in advanced colorectal cancer. N Engl J Med 2008; 359: 1757–1765. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

