Dendrite arborization requires the dynein cofactor NudE
- PMID: 25908857
- PMCID: PMC4450295
- DOI: 10.1242/jcs.170316
Dendrite arborization requires the dynein cofactor NudE
Abstract
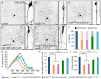
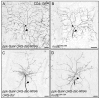
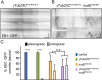
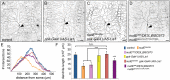
The microtubule-based molecular motor dynein is essential for proper neuronal morphogenesis. Dynein activity is regulated by cofactors, and the role(s) of these cofactors in shaping neuronal structure are still being elucidated. Using Drosophila melanogaster, we reveal that the loss of the dynein cofactor NudE results in abnormal dendrite arborization. Our data show that NudE associates with Golgi outposts, which mediate dendrite branching, suggesting that NudE normally influences dendrite patterning by regulating Golgi outpost transport. Neurons lacking NudE also have increased microtubule dynamics, reflecting a change in microtubule stability that is likely to also contribute to abnormal dendrite growth and branching. These defects in dendritogenesis are rescued by elevating levels of Lis1, another dynein cofactor that interacts with NudE as part of a tripartite complex. Our data further show that the NudE C-terminus is dispensable for dendrite morphogenesis and is likely to modulate NudE activity. We propose that a key function of NudE is to enhance an interaction between Lis1 and dynein that is crucial for motor activity and dendrite architecture.
Keywords: Dendrite patterning; Drosophila; Dynein; Microtubules; Nde1; Ndel1; NudE.
© 2015. Published by The Company of Biologists Ltd.
Conflict of interest statement
The authors declare no competing or financial interests.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

