Management of egfr tki-induced dermatologic adverse events
- PMID: 25908911
- PMCID: PMC4399609
- DOI: 10.3747/co.22.2430
Management of egfr tki-induced dermatologic adverse events
Abstract
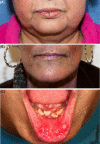
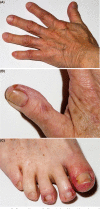
Targeting the epidermal growth factor receptor (egfr) pathway has become standard practice for the treatment of advanced non-small-cell lung cancer. Compared with chemotherapy, egfr tyrosine kinase inhibitors (tkis) have been associated with improved efficacy in patients with an EGFR mutation. Together with the increase in efficacy comes an adverse event (ae) profile different from that of chemotherapy. That profile includes three of the most commonly occurring dermatologic aes: acneiform rash, stomatitis, and paronychia. Currently, no randomized clinical trials have evaluated the treatments for the dermatologic aes that patients experience when taking egfr tkis. Based on the expert opinion of the authors, some basic strategies have been developed to manage those key dermatologic aes. Those strategies have the potential to improve patient quality of life and compliance and to prevent inappropriate dose reductions.
Keywords: Non-small-cell lung cancer; acneiform rash; adverse events; egfr tkis; paronychia; stomatitis.
Figures

References
-
- Fukuoka M, Yano S, Giaccone G, et al. Multi-institutional randomized phase ii trial of gefitinib for previously treated patients with advanced non-small-cell lung cancer (the ideal 1 trial) J Clin Oncol. 2003;21:2237–46. doi: 10.1200/JCO.2003.10.038. [Erratum in: J Clin Oncol 2004;22:4863] - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

