Response to Pneumococcal Polysaccharide Vaccination in Newly Diagnosed HIV-Positive Individuals
- PMID: 25908995
- PMCID: PMC4405239
- DOI: 10.4172/2155-6113.1000419
Response to Pneumococcal Polysaccharide Vaccination in Newly Diagnosed HIV-Positive Individuals
Abstract
Background: Newly diagnosed HIV-positive individuals are 35 to 100-fold more susceptible to Streptococcus pneumoniae infection compared to non-infected individuals. Therefore, the 23-valent pneumococcal polysaccharide vaccine (PPV23) has previously been recommended, though efficacy and effectiveness of vaccination remains controversial. Early severe B cell dysfunction is a central feature of HIV infection. The specific nature of the immune cells involved in the production of protective antigen-specific antibodies in HIV-positive individuals remains to be elucidated.
Objectives: Evaluate the antibody and antigen-specific B cell response to the 23-valent pneumococcal polysaccharide vaccine in newly diagnosed HIV-positive patients. Moreover, determine if newly diagnosed patients with CD4<200 cells/μl benefit from 6-12 months of HAART, allowing partial viral suppression and immune reconstitution, prior to immunization.
Methods: Newly diagnosed HIV-positive patients with CD4>200 cells/μl and CD4<200 cells/μl were immunized with PPV23. Patients with CD4<200 cells/μl received either immediate or delayed immunization following 6-12 months of HAART. Antibody responses, opsonophagocytic activity and phenotypic analysis of pneumococcal polysaccharide-specific B cells were studied.
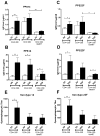
Results: Newly diagnosed HIV-positive patients demonstrated CD4-dependent increases in antibody and opsonophagocytic titers thought to be commensurate with protection. Functional opsonophagocytic titers of patients with CD4<200 cells/μl immunized immediately compared to patients with CD4<200 cells/μl receiving HAART for 6-12 months were not significantly different. Pneumococcal polysaccharide-specific B cells were distributed evenly between IgM memory and switched memory B cells for all groups, but IgM memory B cells were significantly lower than in HIV-negative individuals.
Conclusions: Despite CD4-dependent pneumococcal polysaccharide-specific deficiencies in newly diagnosed HIV-positive patients, vaccination was beneficial based on opsonophagocytic titers for all newly diagnosed HIV-positive groups. In HIV-positive patients with CD4<200 cells/μl, 6-12 months of HAART did not improve opsonophagocytic titers or antibody concentrations. Based on these findings, immunization with the 23-valent pneumococcal polysaccharide vaccine should not be delayed in newly diagnosed HIV-positive patients with CD4<200 cells/μl.
Keywords: Antigen-specific; B cell; HIV; Pneumococcus; Polysaccharide; Streptococcus pneumonia; Vaccine.
Figures
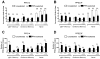
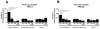
References
-
- Grau I, Pallares R, Tubau F, Schulze MH, Llopis F, et al. Epidemiologic changes in bacteremic pneumococcal disease in patients with human immunodeficiency virus in the era of highly active antiretroviral therapy. Arch Intern Med. 2005;165:1533–1540. - PubMed
-
- Heffernan RT, Barrett NL, Gallagher KM, Hadler JL, Harrison LH, et al. Declining incidence of invasive Streptococcus pneumoniae infections among persons with AIDS in an era of highly active antiretroviral therapy, 1995–2000. J Infect Dis. 2005;191:2038–2045. - PubMed
-
- Janoff EN, Breiman RF, Daley CL, Hopewell PC. Pneumococcal disease during HIV infection. Epidemiologic, clinical, and immunologic perspectives. Ann Intern Med. 1992;117:314–324. - PubMed
-
- Yin Z, Rice BD, Waight P, Miller E, George R, et al. Invasive pneumococcal disease among HIV-positive individuals, 2000–2009. AIDS. 2012;26:87–94. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
