HIF-1α Mediates Isoflurane-Induced Vascular Protection in Subarachnoid Hemorrhage
- PMID: 25909079
- PMCID: PMC4402079
- DOI: 10.1002/acn3.170
HIF-1α Mediates Isoflurane-Induced Vascular Protection in Subarachnoid Hemorrhage
Abstract
Objective: Outcome after aneurysmal subarachnoid hemorrhage (SAH) depends critically on delayed cerebral ischemia (DCI) - a process driven primarily by vascular events including cerebral vasospasm, microvessel thrombosis, and microvascular dysfunction. This study sought to determine the impact of postconditioning - the phenomenon whereby endogenous protection against severe injury is enhanced by subsequent exposure to a mild stressor - on SAH-induced DCI.
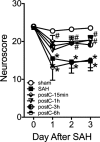
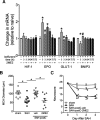
Methods: Adult male C57BL/6 mice were subjected to sham, SAH, or SAH plus isoflurane postconditioning. Neurological outcome was assessed daily via sensorimotor scoring. Contributors to DCI including cerebral vasospasm, microvessel thrombosis, and microvascular dysfunction were measured 3 days later. Isoflurane-induced changes in hypoxia-inducible factor 1alpha (HIF-1α)-dependent genes were assessed via quantitative polymerase chain reaction. HIF-1α was inhibited pharmacologically via 2-methoxyestradiol (2ME2) or genetically via endothelial cell HIF-1α-null mice (EC-HIF-1α-null). All experiments were performed in a randomized and blinded fashion.
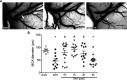
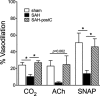
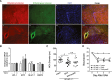
Results: Isoflurane postconditioning initiated at clinically relevant time points after SAH significantly reduced cerebral vasospasm, microvessel thrombosis, microvascular dysfunction, and neurological deficits in wild-type (WT) mice. Isoflurane modulated HIF-1α-dependent genes - changes that were abolished in 2ME2-treated WT mice and EC-HIF-1α-null mice. Isoflurane-induced DCI protection was attenuated in 2ME2-treated WT mice and EC-HIF-1α-null mice.
Interpretation: Isoflurane postconditioning provides strong HIF-1α-mediated macro- and microvascular protection in SAH, leading to improved neurological outcome. These results implicate cerebral vessels as a key target for the brain protection afforded by isoflurane postconditioning, and HIF-1α as a critical mediator of this vascular protection. They also identify isoflurane postconditioning as a promising novel therapeutic for SAH.
Figures

References
-
- Vergouwen MD, Vermeulen M, van Gijn J, et al. Definition of delayed cerebral ischemia after aneurysmal subarachnoid hemorrhage as an outcome event in clinical trials and observational studies: proposal of a multidisciplinary research group. Stroke. 2010;41:2391–2395. - PubMed
-
- Macdonald RL. Delayed neurological deterioration after subarachnoid haemorrhage. Nat Rev Neurol. 2014;10:44–58. - PubMed
-
- Hijdra A, Van Gijn J, Stefanko S, et al. Delayed cerebral ischemia after aneurysmal subarachnoid hemorrhage: clinicoanatomic correlations. Neurology. 1986;36:329–333. - PubMed
-
- Rabinstein AA, Friedman JA, Weigand SD, et al. Predictors of cerebral infarction in aneurysmal subarachnoid hemorrhage. Stroke. 2004;35:1862–1866. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

