Endplate denervation correlates with Nogo-A muscle expression in amyotrophic lateral sclerosis patients
- PMID: 25909082
- PMCID: PMC4402082
- DOI: 10.1002/acn3.179
Endplate denervation correlates with Nogo-A muscle expression in amyotrophic lateral sclerosis patients
Abstract
Objective: Data from mouse models of amyotrophic lateral sclerosis (ALS) suggest early morphological changes in neuromuscular junctions (NMJs), with loss of nerve-muscle contact. Overexpression of the neurite outgrowth inhibitor Nogo-A in muscle may play a role in this loss of endplate innervation.
Methods: We used confocal and electron microscopy to study the structure of the NMJs in muscle samples collected from nine ALS patients (five early-stage patients and four long-term survivors). We correlated the morphological results with clinical and electrophysiological data, and with Nogo-A muscle expression level.
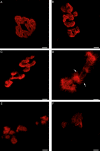
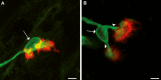
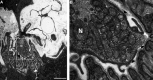
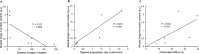
Results: Surface electromyography assessment of neuromuscular transmission was abnormal in 3/9 ALS patients. The postsynaptic apparatus was morphologically altered for almost all NMJs (n = 430) analyzed using confocal microscopy. 19.7% of the NMJs were completely denervated (fragmented synaptic gutters and absence of nerve terminal profile). The terminal axonal arborization was usually sparsely branched and 56.8% of innervated NMJs showed a typical reinnervation pattern. Terminal Schwann cell (TSC) morphology was altered with extensive cytoplasmic processes. A marked intrusion of TSCs in the synaptic cleft was seen in some cases, strikingly reducing the synaptic surface available for neuromuscular transmission. Finally, high-level expression of Nogo-A in muscle was significantly associated with higher extent of NMJ denervation and negative functional outcome.
Interpretation: Our results support the hypothesis that morphological alterations of NMJs are present from early-stage disease and may significantly contribute to functional motor impairment in ALS patients. Muscle expression of Nogo-A is associated with NMJ denervation and thus constitutes a therapeutic target to slow disease progression.
Figures


References
-
- Charcot JM, Joffroy A. Deux cas d'atrophie musculaire progressive avec lésions de la substance grise et des faisceaux antéro-latéraux de la moelle épinière. Arch Physiol. 1869;2:354–367.
-
- Beghi E, Mennini T, Bendotti C, et al. The heterogeneity of amyotrophic lateral sclerosis: a possible explanation of treatment failure. Curr Med Chem. 2007;14:3185–3200. - PubMed
-
- Fischer LR, Culver DG, Tennant P, et al. Amyotrophic lateral sclerosis is a distal axonopathy: evidence in mice and man. Exp Neurol. 2004;185:232–240. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

