Blocking GluR2-GAPDH ameliorates experimental autoimmune encephalomyelitis
- PMID: 25909084
- PMCID: PMC4402084
- DOI: 10.1002/acn3.182
Blocking GluR2-GAPDH ameliorates experimental autoimmune encephalomyelitis
Abstract
Objective: Multiple sclerosis (MS) is the most common disabling neurological disease of young adults. The pathophysiological mechanism of MS remains largely unknown and no cure is available. Current clinical treatments for MS modulate the immune system, with the rationale that autoimmunity is at the core of MS pathophysiology.
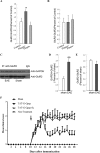
Methods: Experimental autoimmune encephalitis (EAE) was induced in mice with MOG35-55 and clinical scoring was performed to monitor signs of paralysis. EAE mice were injected intraperitoneally with TAT-fusion peptides daily from day 10 until day 30 after immunization, and their effects were measured at day 17 or day 30.
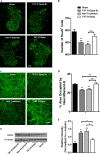
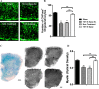
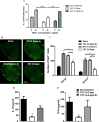
Results: We report a novel target for the development of MS therapy, which aimed at blocking glutamate-mediated neurotoxicity through targeting the interaction between the AMPA (2-amino-3-(3-hydroxy-5-methyl-isoxazol-4-yl) propanoic acid) receptor and an interacting protein. We found that protein complex composed of the GluR2 subunit of AMPA receptors and GAPDH (glyceraldehyde-3-phosphate dehydrogenase) was present at significantly higher levels in postmortem tissue from MS patients and in EAE mice, an animal model for MS. Next, we developed a peptide that specifically disrupts the GluR2 -GAPDH complex. This peptide greatly improves neurological function in EAE mice, reduces neuron death, rescues demyelination, increases oligodendrocyte survival, and reduces axonal damage in the spinal cords of EAE mice. More importantly, our peptide has no direct suppressive effect on naive T-cell responses or basal neurotransmission.
Interpretation: The GluR2 -GAPDH complex represents a novel therapeutic target for the development of medications for MS that work through a different mechanism than existing treatments.
Figures
References
-
- Trapp BD, Stys PK. Virtual hypoxia and chronic necrosis of demyelinated axons in multiple sclerosis. Lancet Neurol. 2009;8:280–291. - PubMed
-
- Compston A, Coles A. Multiple sclerosis. Lancet. 2008;372:1502–1517. - PubMed
-
- Kieseier BC, Wiendl H, Hemmer B, Hartung HP. Treatment and treatment trials in multiple sclerosis. Curr Opin Neurol. 2007;20:286–293. - PubMed
-
- Trapp BD, Nave KA. Multiple sclerosis: an immune or neurodegenerative disorder? Annu Rev Neurosci. 2008;31:247–269. - PubMed
-
- Stys PK, Zamponi GW, van Minnen J, Geurts JJ. Will the real multiple sclerosis please stand up? Nat Rev Neurosci. 2012;13:507–514. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

