MicroRNA let-7c Inhibits Cell Proliferation and Induces Cell Cycle Arrest by Targeting CDC25A in Human Hepatocellular Carcinoma
- PMID: 25909324
- PMCID: PMC4409336
- DOI: 10.1371/journal.pone.0124266
MicroRNA let-7c Inhibits Cell Proliferation and Induces Cell Cycle Arrest by Targeting CDC25A in Human Hepatocellular Carcinoma
Retraction in
-
Retraction: MicroRNA let-7c Inhibits Cell Proliferation and Induces Cell Cycle Arrest by Targeting CDC25A in Human Hepatocellular Carcinoma.PLoS One. 2023 Jan 6;18(1):e0280394. doi: 10.1371/journal.pone.0280394. eCollection 2023. PLoS One. 2023. PMID: 36607989 Free PMC article. No abstract available.
Abstract
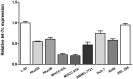
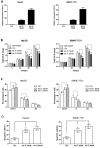
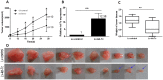
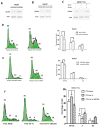
Down-regulation of the microRNA let-7c plays an important role in the pathogenesis of human hepatocellular carcinoma (HCC). The aim of the present study was to determine whether the cell cycle regulator CDC25A is involved in the antitumor effect of let-7c in HCC. The expression levels of let-7c in HCC cell lines were examined by quantitative real-time PCR, and a let-7c agomir was transfected into HCC cells to overexpress let-7c. The effects of let-7c on HCC proliferation, apoptosis and cell cycle were analyzed. The in vivo tumor-inhibitory efficacy of let-7c was evaluated in a xenograft mouse model of HCC. Luciferase reporter assays and western blotting were conducted to identify the targets of let-7c and to determine the effects of let-7c on CDC25A, CyclinD1, CDK6, pRb and E2F2 expression. The results showed that the expression levels of let-7c were significantly decreased in HCC cell lines. Overexpression of let-7c repressed cell growth, induced cell apoptosis, led to G1 cell cycle arrest in vitro, and suppressed tumor growth in a HepG2 xenograft model in vivo. The luciferase reporter assay showed that CDC25A was a direct target of let-7c, and that let-7c inhibited the expression of CDC25A protein by directly targeting its 3' UTR. Restoration of CDC25A induced a let-7c-mediated G1-to-S phase transition. Western blot analysis demonstrated that overexpression of let-7c decreased CyclinD1, CDK6, pRb and E2F2 protein levels. In conclusion, this study indicates that let-7c suppresses HCC progression, possibly by directly targeting the cell cycle regulator CDC25A and indirectly affecting its downstream target molecules. Let-7c may therefore be an effective therapeutic target for HCC.
Conflict of interest statement
Figures



References
-
- Bartel DP. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell. 2004; 116: 281–297. - PubMed
-
- Ambros V. The functions of animal microRNAs. Nature. 2004; 431: 350–355. - PubMed
-
- He L, Hannon GJ. MicroRNAs: small RNAs with a big role in gene regulation. Nat Rev Genet. 2004; 5: 522–531. - PubMed
-
- Kloosterman WP, Plasterk RH. The diverse functions of microRNAs in animal development and disease. Dev Cell. 2006; 11: 441–450. - PubMed
-
- Calin GA, Croce CM. MicroRNA signatures in human cancers. Nat Rev Cancer. 2006; 6: 857–866. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

