Chaperone-like protein p32 regulates ULK1 stability and autophagy
- PMID: 25909887
- PMCID: PMC4648329
- DOI: 10.1038/cdd.2015.34
Chaperone-like protein p32 regulates ULK1 stability and autophagy
Abstract
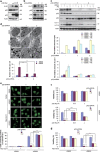
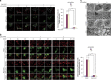
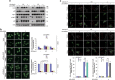
Mitophagy mediates clearance of dysfunctional mitochondria, and represents one type of mitochondrial quality control, which is essential for optimal mitochondrial bioenergetics. p32, a chaperone-like protein, is crucial for maintaining mitochondrial membrane potential and oxidative phosphorylation. However, the relationship between p32 and mitochondrial homeostasis has not been addressed. Here, we identified p32 as a key regulator of ULK1 stability by forming complex with ULK1. p32 depletion potentiated K48-linked but impaired K63-linked polyubiquitination of ULK1, leading to proteasome-mediated degradation of ULK1. As a result, silencing p32 profoundly impaired starvation-induced autophagic flux and the clearance of damaged mitochondria caused by mitochondrial uncoupler. Importantly, restoring ULK1 expression in p32-depleted cells rescued autophagy and mitophagy defects. Our findings highlight a cytoprotective role of p32 under starvation conditions by regulating ULK1 stability, and uncover a crucial role of the p32-ULK1-autophagy axis in coordinating stress response, cell survival and mitochondrial homeostasis.
Figures



References
-
- 2Matthews DA, Russell WC. Adenovirus core protein V interacts with p32—a protein which is associated with both the mitochondria and the nucleus. J Gen Virol 1998; 79: 1677–1685. - PubMed
-
- 4Soltys BJ, Kang D, Gupta RS. Localization of P32 protein (gC1q-R) in mitochondria and at specific extramitochondrial locations in normal tissues. Histochem Cell Biol 2000; 114: 245–255. - PubMed
-
- 5van Leeuwen HC, O'Hare P. Retargeting of the mitochondrial protein p32/gC1Qr to a cytoplasmic compartment and the cell surface. J Cell Sci 2001; 114: 2115–2123. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

