C-terminal threonines and serines play distinct roles in the desensitization of rhodopsin, a G protein-coupled receptor
- PMID: 25910054
- PMCID: PMC4438306
- DOI: 10.7554/eLife.05981
C-terminal threonines and serines play distinct roles in the desensitization of rhodopsin, a G protein-coupled receptor
Abstract
Rod photoreceptors generate measurable responses to single-photon activation of individual molecules of the G protein-coupled receptor (GPCR), rhodopsin. Timely rhodopsin desensitization depends on phosphorylation and arrestin binding, which quenches G protein activation. Rhodopsin phosphorylation has been measured biochemically at C-terminal serine residues, suggesting that these residues are critical for producing fast, low-noise responses. The role of native threonine residues is unclear. We compared single-photon responses from rhodopsin lacking native serine or threonine phosphorylation sites. Contrary to expectation, serine-only rhodopsin generated prolonged step-like single-photon responses that terminated abruptly and randomly, whereas threonine-only rhodopsin generated responses that were only modestly slower than normal. We show that the step-like responses of serine-only rhodopsin reflect slow and stochastic arrestin binding. Thus, threonine sites play a privileged role in promoting timely arrestin binding and rhodopsin desensitization. Similar coordination of phosphorylation and arrestin binding may more generally permit tight control of the duration of GPCR activity.
Keywords: G-protein-coupled receptor; G-protein-coupled receptor kinase; arrestin; biophysics; mouse; neuroscience; single-photon response; structural biology.
Conflict of interest statement
The authors declare that no competing interests exist.
Figures
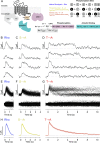

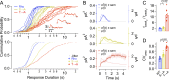


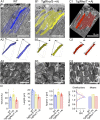
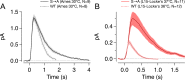
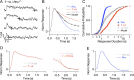

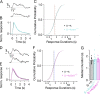

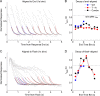
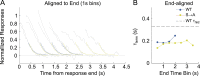
References
-
- Ascano MT, Smith WC, Gregurick SK, Robinson PR. Arrestin residues involved in the functional binding of arrestin to phosphorylated, photolyzed rhodopsin. Molecular Vision. 2006;12:1516–1525. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- HL086865/HL/NHLBI NIH HHS/United States
- HL071818/HL/NHLBI NIH HHS/United States
- T32GM-07270/GM/NIGMS NIH HHS/United States
- EY11500/EY/NEI NIH HHS/United States
- R01 HL086865/HL/NHLBI NIH HHS/United States
- T32 GM007270/GM/NIGMS NIH HHS/United States
- P30 EY001730/EY/NEI NIH HHS/United States
- R01 EY011850/EY/NEI NIH HHS/United States
- EY11850/EY/NEI NIH HHS/United States
- Howard Hughes Medical Institute/United States
- R01 EY012155/EY/NEI NIH HHS/United States
- T32 EY007031/EY/NEI NIH HHS/United States
- R01 EY011500/EY/NEI NIH HHS/United States
- R01 HL071818/HL/NHLBI NIH HHS/United States
- T32EY-07031/EY/NEI NIH HHS/United States
- EY12155/EY/NEI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

