Selective elimination of mitochondrial mutations in the germline by genome editing
- PMID: 25910206
- PMCID: PMC4505837
- DOI: 10.1016/j.cell.2015.03.051
Selective elimination of mitochondrial mutations in the germline by genome editing
Abstract
Mitochondrial diseases include a group of maternally inherited genetic disorders caused by mutations in mtDNA. In most of these patients, mutated mtDNA coexists with wild-type mtDNA, a situation known as mtDNA heteroplasmy. Here, we report on a strategy toward preventing germline transmission of mitochondrial diseases by inducing mtDNA heteroplasmy shift through the selective elimination of mutated mtDNA. As a proof of concept, we took advantage of NZB/BALB heteroplasmic mice, which contain two mtDNA haplotypes, BALB and NZB, and selectively prevented their germline transmission using either mitochondria-targeted restriction endonucleases or TALENs. In addition, we successfully reduced human mutated mtDNA levels responsible for Leber's hereditary optic neuropathy (LHOND), and neurogenic muscle weakness, ataxia, and retinitis pigmentosa (NARP), in mammalian oocytes using mitochondria-targeted TALEN (mito-TALENs). Our approaches represent a potential therapeutic avenue for preventing the transgenerational transmission of human mitochondrial diseases caused by mutations in mtDNA. PAPERCLIP.
Copyright © 2015 Elsevier Inc. All rights reserved.
Figures
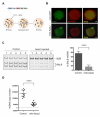
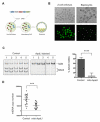
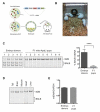
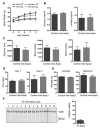
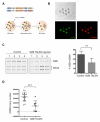
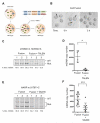
Comment in
-
Converting a Problem into an Opportunity: mtDNA Heteroplasmy Shift.Cell Stem Cell. 2015 May 7;16(5):457-8. doi: 10.1016/j.stem.2015.04.012. Cell Stem Cell. 2015. PMID: 25957899
References
-
- Anderson S, Bankier AT, Barrell BG, Debruijn M, Coulson AR, Drouin J, Eperon IC, Nierlich DP, Roe BA, Sanger F, et al. Sequence and Organization of the Human Mitochondrial Genome. Nature. 1981;290:457–465. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

