Small molecule fluoride toxicity agonists
- PMID: 25910244
- PMCID: PMC4617673
- DOI: 10.1016/j.chembiol.2015.03.016
Small molecule fluoride toxicity agonists
Abstract
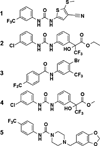
Fluoride is a ubiquitous anion that inhibits a wide variety of metabolic processes. Here, we report the identification of a series of compounds that enhance fluoride toxicity in Escherichia coli and Streptococcus mutans. These molecules were isolated by using a high-throughput screen (HTS) for compounds that increase intracellular fluoride levels as determined via a fluoride riboswitch reporter fusion construct. A series of derivatives were synthesized to examine structure-activity relationships, leading to the identification of compounds with improved activity. Thus, we demonstrate that small molecule fluoride toxicity agonists can be identified by HTS from existing chemical libraries by exploiting a natural fluoride riboswitch. In addition, our findings suggest that some molecules might be further optimized to function as binary antibacterial agents when combined with fluoride.
Copyright © 2015 Elsevier Ltd. All rights reserved.
Figures




Similar articles
-
Employing a ZTP Riboswitch to Detect Bacterial Folate Biosynthesis Inhibitors in a Small Molecule High-Throughput Screen.ACS Chem Biol. 2019 Dec 20;14(12):2841-2850. doi: 10.1021/acschembio.9b00713. Epub 2019 Nov 14. ACS Chem Biol. 2019. PMID: 31609568 Free PMC article.
-
Gramicidin D enhances the antibacterial activity of fluoride.Bioorg Med Chem Lett. 2014 Jul 1;24(13):2969-2971. doi: 10.1016/j.bmcl.2014.03.061. Epub 2014 Mar 28. Bioorg Med Chem Lett. 2014. PMID: 24857543 Free PMC article.
-
Effects of two fluoride varnishes and one fluoride/chlorhexidine varnish on Streptococcus mutans and Streptococcus sobrinus biofilm formation in vitro.Int J Med Sci. 2012;9(2):129-36. doi: 10.7150/ijms.3637. Epub 2012 Jan 7. Int J Med Sci. 2012. PMID: 22253559 Free PMC article.
-
Excellent fluoride decontamination and antibacterial efficacy of Fe-Ca-Zr hybrid metal oxide nanomaterial.J Colloid Interface Sci. 2015 Nov 1;457:289-97. doi: 10.1016/j.jcis.2015.06.045. Epub 2015 Jun 30. J Colloid Interface Sci. 2015. PMID: 26196712 Review.
-
Progress of Antimicrobial Discovery Against the Major Cariogenic Pathogen Streptococcus mutans.Curr Issues Mol Biol. 2019;32:601-644. doi: 10.21775/cimb.032.601. Epub 2019 Jun 5. Curr Issues Mol Biol. 2019. PMID: 31166181 Review.
Cited by
-
Employing a ZTP Riboswitch to Detect Bacterial Folate Biosynthesis Inhibitors in a Small Molecule High-Throughput Screen.ACS Chem Biol. 2019 Dec 20;14(12):2841-2850. doi: 10.1021/acschembio.9b00713. Epub 2019 Nov 14. ACS Chem Biol. 2019. PMID: 31609568 Free PMC article.
-
An in vivo high-throughput screening for riboswitch ligands using a reverse reporter gene system.Sci Rep. 2017 Aug 10;7(1):7732. doi: 10.1038/s41598-017-07870-w. Sci Rep. 2017. PMID: 28798404 Free PMC article.
-
A bacterial riboswitch class senses xanthine and uric acid to regulate genes associated with purine oxidation.RNA. 2020 Aug;26(8):960-968. doi: 10.1261/rna.075218.120. Epub 2020 Apr 28. RNA. 2020. PMID: 32345632 Free PMC article.
-
Metalloriboswitches: RNA-based inorganic ion sensors that regulate genes.J Biol Chem. 2017 Jun 9;292(23):9441-9450. doi: 10.1074/jbc.R117.787713. Epub 2017 Apr 28. J Biol Chem. 2017. PMID: 28455443 Free PMC article. Review.
-
Novel urea derivative-loaded PLGA nanoparticles to inhibit caries-associated Streptococcus mutans.RSC Adv. 2022 Feb 2;12(7):4072-4080. doi: 10.1039/d1ra09314b. eCollection 2022 Jan 28. RSC Adv. 2022. PMID: 35425421 Free PMC article.
References
-
- Barbier O, Arreola-Mendoza L, Del Razo LM. Molecular mechanisms of fluoride toxicity. Chem. Biol. Interact. 2010;188:319–333. - PubMed
-
- Boiocchi M, Del Boca L, Gomez DE, Fabbrizzi L, Licchelli M, Monzani E. Nature of urea-fluoride interaction: incipient and definitive proton transfer. J. Am. Chem. Soc. 2004;126:16507–16514. - PubMed
-
- Brooks SJ, Edwards PR, Gale PA, Light ME. Carboxylate complexation by a family of easy-to-make ortho-phenylenediamine based bis-ureas: studies in solution and the solid state. New J. Chem. 2006;30:65–70.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

