Circulating mitochondrial DNA and Toll-like receptor 9 are associated with vascular dysfunction in spontaneously hypertensive rats
- PMID: 25910936
- PMCID: PMC4560046
- DOI: 10.1093/cvr/cvv137
Circulating mitochondrial DNA and Toll-like receptor 9 are associated with vascular dysfunction in spontaneously hypertensive rats
Abstract
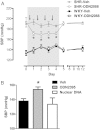
Aims: Immune system activation is a common feature of hypertension pathogenesis. However, the mechanisms that initiate this activation are not well understood. Innate immune system recognition and response to danger are becoming apparent in many cardiovascular diseases. Danger signals can arise from not only pathogens, but also damage-associated molecular patterns (DAMPs). Our first hypothesis was that the DAMP, mitochondrial DNA (mtDNA), which is recognized by Toll-like receptor 9 (TLR9), is elevated in the circulation of spontaneously hypertensive rats (SHR), and that the deoxyribonuclease enzymes responsible for its degradation have decreased activity in SHR. Based on these novel SHR phenotypes, we further hypothesized that (i) treatment of SHR with an inhibitory oligodinucleotide for TLR9 (ODN2088) would lower blood pressure and that (ii) treatment of normotensive rats with a TLR9-specific CpG oligonucleotide (ODN2395) would cause endothelial dysfunction and increase blood pressure.
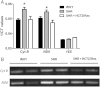
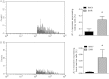
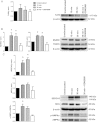
Methods and results: We observed that SHR have elevated circulating mtDNA and diminished deoxyribonuclease I and II activity. Additionally, treatment of SHR with ODN2088 lowered systolic blood pressure. On the other hand, treatment of normotensive rats with ODN2395 increased systolic blood pressure and rendered their arteries less sensitive to acetylcholine-induced relaxation and more sensitive to norepinephrine-induced contraction. This dysfunctional vasoreactivity was due to increased cyclooxygenase and p38 mitogen-activated protein kinase activation, increased reactive oxygen species generation, and reduced nitric oxide bioavailability.
Conclusion: Circulating mtDNA and impaired deoxyribonuclease activity may lead to the activation of the innate immune system, via TLR9, and contribute to elevated arterial pressure and vascular dysfunction in SHR.
Keywords: Hypertension; Innate immunity; Mitochondrial DNA; Toll-like receptor 9; Vascular dysfunction.
Published on behalf of the European Society of Cardiology. All rights reserved. © The Author 2015. For permissions please email: journals.permissions@oup.com.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

