Patient-reported outcomes in randomised controlled trials of colorectal cancer: an analysis determining the availability of robust data to inform clinical decision-making
- PMID: 25910987
- PMCID: PMC4784226
- DOI: 10.1007/s00432-015-1970-x
Patient-reported outcomes in randomised controlled trials of colorectal cancer: an analysis determining the availability of robust data to inform clinical decision-making
Abstract
Purpose: Randomised controlled trials (RCTs) are the most robust study design measuring outcomes of colorectal cancer (CRC) treatments, but to influence clinical practice trial design and reporting of patient-reported outcomes (PROs) must be of high quality. Objectives of this study were as follows: to examine the quality of PRO reporting in RCTs of CRC treatment; to assess the availability of robust data to inform clinical decision-making; and to investigate whether quality of reporting improved over time.
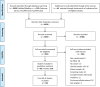
Methods: A systematic review from January 2004-February 2012 identified RCTs of CRC treatment describing PROs. Relevant abstracts were screened and manuscripts obtained. Methodological quality was assessed using International Society for Quality of Life Research-patient-reported outcome reporting standards. Changes in reporting quality over time were established by comparison with previous data, and risk of bias was assessed with the Cochrane risk of bias tool.
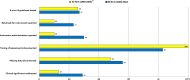
Results: Sixty-six RCTs were identified, seven studies (10 %) reported survival benefit favouring the experimental treatment, 35 trials (53 %) identified differences in PROs between treatment groups, and the clinical significance of these differences was discussed in 19 studies (29 %). The most commonly reported treatment type was chemotherapy (n = 45; 68 %). Improvements over time in key methodological issues including the documentation of missing data and the discussion of the clinical significance of PROs were found. Thirteen trials (20 %) had high-quality reporting.
Conclusions: Whilst improvements in PRO quality reporting over time were found, several recent studies still fail to robustly inform clinical practice. Quality of PRO reporting must continue to improve to maximise the clinical impact of PRO findings.
Keywords: Cancer; Colorectal; Health-related quality of life; Patient-reported outcomes; Randomised; Trails.
Conflict of interest statement
None.
Figures

References
-
- Bauhofer A et al (2007) Perioperative prophylaxis with granulocyte colony-stimulating factor (G-CSF) in high-risk colorectal cancer patients for an improved recovery: a randomized, controlled trial. Surgery 141:501–510. doi:10.1016/j.surg.2006.09.004 - PubMed
-
- Calvert M, Blazeby J, Altman DG, Revicki DA, Moher D, Brundage MD, Group CP (2013) Reporting of patient-reported outcomes in randomized trials: the CONSORT PRO extension. JAMA 309:814–822. doi:10.1001/jama.2013.879 - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

