Redox Control of Protein Arginine Methyltransferase 1 (PRMT1) Activity
- PMID: 25911106
- PMCID: PMC4463439
- DOI: 10.1074/jbc.M115.651380
Redox Control of Protein Arginine Methyltransferase 1 (PRMT1) Activity
Abstract
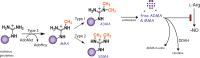
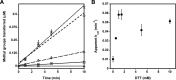
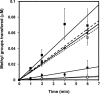
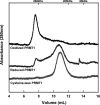
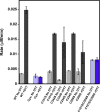
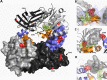
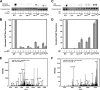
Elevated levels of asymmetric dimethylarginine (ADMA) correlate with risk factors for cardiovascular disease. ADMA is generated by the catabolism of proteins methylated on arginine residues by protein arginine methyltransferases (PRMTs) and is degraded by dimethylarginine dimethylaminohydrolase. Reports have shown that dimethylarginine dimethylaminohydrolase activity is down-regulated and PRMT1 protein expression is up-regulated under oxidative stress conditions, leading many to conclude that ADMA accumulation occurs via increased synthesis by PRMTs and decreased degradation. However, we now report that the methyltransferase activity of PRMT1, the major PRMT isoform in humans, is impaired under oxidative conditions. Oxidized PRMT1 displays decreased activity, which can be rescued by reduction. This oxidation event involves one or more cysteine residues that become oxidized to sulfenic acid (-SOH). We demonstrate a hydrogen peroxide concentration-dependent inhibition of PRMT1 activity that is readily reversed under physiological H2O2 concentrations. Our results challenge the unilateral view that increased PRMT1 expression necessarily results in increased ADMA synthesis and demonstrate that enzymatic activity can be regulated in a redox-sensitive manner.
Keywords: ADMA; PRMT1; arginine methylation; endothelial dysfunction; hydrogen peroxide; oxidative stress; posttranslational modification (PTM); protein arginine methyltransferase; redox regulation; sulfenic acid.
© 2015 by The American Society for Biochemistry and Molecular Biology, Inc.
Figures


References
-
- Go A. S., Mozaffarian D., Roger V. L., Benjamin E. J., Berry J. D., Blaha M. J., Dai S., Ford E. S., Fox C. S., Franco S., Fullerton H. J., Gillespie C., Hailpern S. M., Heit J. A., Howard V. J., Huffman M. D., Judd S. E., Kissela B. M., Kittner S. J., Lackland D. T., Lichtman J. H., Lisabeth L. D., Mackey R. H., Magid D. J., Marcus G. M., Marelli A., Matchar D. B., McGuire D. K., Mohler E. R., 3rd, Moy C. S., Mussolino M. E., Neumar R. W., Nichol G., Pandey D. K., Paynter N. P., Reeves M. J., Sorlie P. D., Stein J., Towfighi A., Turan T. N., Virani S. S., Wong N. D., Woo D., Turner M. B., American Heart Association Statistics Committee and Stroke Statistics Subcommittee (2014) Heart Disease and Stroke Statistics-2014 Update A Report From the American Heart Association. Circulation 129, e28–e292 - PMC - PubMed
-
- Chen X., Niroomand F., Liu Z., Zankl A., Katus H. A., Jahn L., Tiefenbacher C. P. (2006) Expression of nitric oxide related enzymes in coronary heart disease. Basic Res. Cardiol. 101, 346–353 - PubMed
-
- Antoniades C., Shirodaria C., Leeson P., Antonopoulos A., Warrick N., Van-Assche T., Cunnington C., Tousoulis D., Pillai R., Ratnatunga C., Stefanadis C., Channon K. M. (2009) Association of plasma asymmetrical dimethylarginine (ADMA) with elevated vascular superoxide production and endothelial nitric oxide synthase uncoupling: implications for endothelial function in human atherosclerosis. Eur. Heart J. 30, 1142–1150 - PubMed
-
- Böger R. H., Bode-Böger S. M., Szuba A., Tsao P. S., Chan J. R., Tangphao O., Blaschke T. F., Cooke J. P. (1998) Asymmetric dimethylarginine (ADMA): a novel risk factor for endothelial dysfunction: its role in hypercholesterolemia. Circulation 98, 1842–1847 - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
LinkOut - more resources
Full Text Sources

