B7H6-Specific Bispecific T Cell Engagers Lead to Tumor Elimination and Host Antitumor Immunity
- PMID: 25911747
- PMCID: PMC4433849
- DOI: 10.4049/jimmunol.1402517
B7H6-Specific Bispecific T Cell Engagers Lead to Tumor Elimination and Host Antitumor Immunity
Abstract
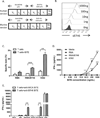
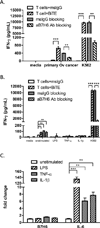
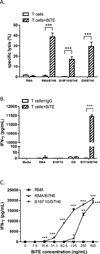
Substantial evidence showed that T cells are the key effectors in immune-mediated tumor eradication; however, most T cells do not exhibit antitumor specificity. In this study, a bispecific T cell engager (BiTE) approach was used to direct T cells to recognize B7H6(+) tumor cells. B7H6 is a specific ligand for the NK cell-activating receptor NKp30. B7H6 is expressed on various types of primary human tumors, including leukemia, lymphoma, and gastrointestinal stromal tumors, but it is not constitutively expressed on normal tissues. Data from this study showed that B7H6-specific BiTEs direct T cells to mediate cellular cytotoxicity and IFN-γ secretion upon coculturing with B7H6(+) tumors. Furthermore, B7H6-specific BiTE exhibited no self-reactivity to proinflammatory monocytes. In vivo, B7H6-specific BiTE greatly enhanced the survival benefit of RMA/B7H6 lymphoma-bearing mice through perforin and IFN-γ effector mechanisms. In addition, long-term survivor mice were protected against an RMA lymphoma tumor rechallenge. The B7H6-specific BiTE therapy also decreased tumor burden in murine melanoma and ovarian cancer models. In conclusion, B7H6-specific BiTE activates host T cells and has the potential to treat various B7H6(+) hematological and solid tumors.
Copyright © 2015 by The American Association of Immunologists, Inc.
Figures


References
-
- Baeuerle PA, Reinhardt C. Bispecific T-cell engaging antibodies for cancer therapy. Cancer Res. 2009;69:4941–4944. - PubMed
-
- Hoffmann P, Hofmeister R, Brischwein K, Brandl C, Crommer S, Bargou R, Itin C, Prang N, Baeuerle PA. Serial killing of tumor cells by cytotoxic T cells redirected with a CD19-/CD3-bispecific single-chain antibody construct. Int J Cancer. 2005;115:98–104. - PubMed
-
- Offner S, Hofmeister R, Romaniuk A, Kufer P, Baeuerle PA. Induction of regular cytolytic T cell synapses by bispecific single-chain antibody constructs on MHC class I-negative tumor cells. Mol Immunol. 2006;43:763–771. - PubMed
-
- Bargou R, Leo E, Zugmaier G, Klinger M, Goebeler M, Knop S, Noppeney R, Viardot A, Hess G, Schuler M, Einsele H, Brandl C, Wolf A, Kirchinger P, Klappers P, Schmidt M, Riethmuller G, Reinhardt C, Baeuerle PA, Kufer P. Tumor regression in cancer patients by very low doses of a T cell-engaging antibody. Science. 2008;321:974–977. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

