Induced autoprocessing of the cytopathic Makes caterpillars floppy-like effector domain of the Vibrio vulnificus MARTX toxin
- PMID: 25912102
- PMCID: PMC4583801
- DOI: 10.1111/cmi.12451
Induced autoprocessing of the cytopathic Makes caterpillars floppy-like effector domain of the Vibrio vulnificus MARTX toxin
Abstract
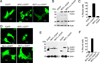
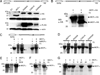
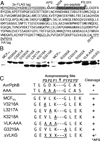
The multifunctional-autoprocessing repeats-in-toxin (MARTX(Vv)) toxin that harbours a varied repertoire of effector domains is the primary virulence factor of Vibrio vulnificus. Although ubiquitously present among Biotype I toxin variants, the 'Makes caterpillars floppy-like' effector domain (MCF(Vv)) is previously unstudied. Using transient expression and protein delivery, MCF(Vv) and MCF(Ah) from the Aeromonas hydrophila MARTX(Ah)) toxin are shown for the first time to induce cell rounding. Alanine mutagenesis across the C-terminal subdomain of MCF(Vv) identified an Arg-Cys-Asp (RCD) tripeptide motif shown to comprise a cysteine protease catalytic site essential for autoprocessing of MCF(Vv). The autoprocessing could be recapitulated in vitro by the addition of host cell lysate to recombinant MCF(Vv), indicating induced autoprocessing by cellular factors. The RCD motif is also essential for cytopathicity, suggesting autoprocessing is essential first to activate the toxin and then to process a cellular target protein resulting in cell rounding. Sequence homology places MCF(Vv) within the C58 cysteine protease family that includes the type III secretion effectors YopT from Yersinia spp. and AvrPphB from Pseudomonas syringae. However, the catalytic site RCD motif is unique compared with other C58 peptidases and is here proposed to represent a new subgroup of autopeptidase found within a number of putative large bacterial toxins.
© 2015 John Wiley & Sons Ltd.
Figures





References
-
- Chung KJ, Cho EJ, Kim MK, Kim YR, Kim SH, Yang HY, et al. RtxA1-induced expression of the small GTPase Rac2 plays a key role in the pathogenicity of Vibrio vulnificus. J Infect Dis. 2010;201:97–105. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

