A conserved MST1/2-YAP axis mediates Hippo signaling during lung growth
- PMID: 25912685
- PMCID: PMC4469623
- DOI: 10.1016/j.ydbio.2015.04.014
A conserved MST1/2-YAP axis mediates Hippo signaling during lung growth
Abstract
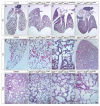
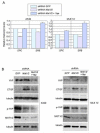
Hippo signaling is a critical player in controlling the growth of several tissues and organs in diverse species. The current model of Hippo signaling postulates a cascade of kinase activity initiated by the MST1/2 kinases in response to external stimuli. This leads to inactivation of the transcriptional coactivators, YAP/TAZ, due to their cytoplasmic retention and degradation that is correlated with YAP/TAZ phosphorylation. In most tissues examined, YAP plays a more dominant role than TAZ. Whether a conserved Hippo pathway is utilized during lung growth and development is unclear. In particular, the regulatory relationship between MST1/2 and YAP/TAZ in the lung remains controversial. By employing the Shh-Cre mouse line to efficiently inactivate genes in the lung epithelium, we show that loss of MST1/2 kinases in the epithelium can lead to neonatal lethality caused by lung defects. This is manifested by perturbation of lung epithelial cell proliferation and differentiation. These phenotypes are more severe than those produced by Nkx2.1-Cre, highlighting the effects of differential Cre activity on phenotypic outcomes. Importantly, expression of YAP targets is upregulated and the ratio of phospho-YAP to total YAP protein levels is reduced in Mst1/2-deficient lungs, all of which are consistent with a negative role of MST1/2 in controlling YAP function. This model gains further support from both in vivo and in vitro studies. Genetic removal of one allele of Yap or one copy of both Yap and Taz rescues neonatal lethality and lung phenotypes due to loss of Mst1/2. Moreover, knockdown of Yap in lung epithelial cell lines restores diminished alveolar marker expression caused by Mst1/2 inactivation. These results demonstrate that MST1/2 inhibit YAP/TAZ activity and establish a conserved MST1/2-YAP axis in coordinating lung growth during development.
Keywords: Development; Hippo signaling; Lung; Mst1/2; Yap.
Copyright © 2015 Elsevier Inc. All rights reserved.
Figures





References
-
- Barry ER, Camargo FD. The Hippo superhighway: signaling crossroads converging on the Hippo/Yap pathway in stem cells and development. Current opinion in cell biology. 2013;25:247–253. - PubMed
-
- Borok Z, Whitsett JA, Bitterman PB, Thannickal VJ, Kotton DN, Reynolds SD, Krasnow MA, Bianchi DW, Morrisey EE, Hogan BL, Kurie JM, Walker DC, Radisky DC, Nishimura SL, Violette SM, Noble PW, Shapiro SD, Blaisdell CJ, Chapman HA, Kiley J, Gail D, Hoshizaki D. Cell plasticity in lung injury and repair: report from an NHLBI workshop, April 19-20, 2010. Proc Am Thorac Soc. 2011;8:215–222. - PMC - PubMed
-
- Chan EH, Nousiainen M, Chalamalasetty RB, Schafer A, Nigg EA, Sillje HH. The Ste20-like kinase Mst2 activates the human large tumor suppressor kinase Lats1. Oncogene. 2005;24:2076–2086. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

