Everolimus and sirolimus in transplantation-related but different
- PMID: 25912929
- PMCID: PMC6053318
- DOI: 10.1517/14740338.2015.1040388
Everolimus and sirolimus in transplantation-related but different
Abstract
Introduction: The inhibitors of the mammalian target of rapamycin (mTOR) sirolimus and everolimus are used not only as immunosuppressants after organ transplantation in combination with calcineurin inhibitors (CNIs) but also as proliferation signal inhibitors coated on drug-eluting stents and in cancer therapy. Notwithstanding their related chemical structures, both have distinct pharmacokinetic, pharmacodynamic and toxicodynamic properties.
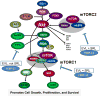
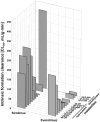
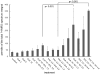
Areas covered: The additional hydroxyethyl group at the C(40) of the everolimus molecule results in different tissue and subcellular distribution, different affinities to active drug transporters and drug-metabolizing enzymes as well as differences in drug-target protein interactions including a much higher potency in terms of interacting with the mTOR complex 2 than sirolimus. Said mechanistic differences as well as differences found in clinical trials in transplant patients are reviewed.
Expert opinion: In comparison to sirolimus, everolimus has higher bioavailability, a shorter terminal half-life, different blood metabolite patterns, the potential to antagonize the negative effects of CNIs on neuronal and kidney cell metabolism (which sirolimus enhances), the ability to stimulate mitochondrial oxidation (which sirolimus inhibits) and to reduce vascular inflammation to a greater extent. A head-to-head, randomized trial comparing the safety and tolerability of these two mTOR inhibitors in solid organ transplant recipients is merited.
Keywords: comparison; drug metabolism; everolimus; mammalian target of rapamycin complex 2; mitochondria; nephrotoxicity; neuronal metabolism; pharmacokinetics; sirolimus; vascular inflammation.
Conflict of interest statement
BN has received research funding and honoraria from Novartis Pharma and has served on Novartis Pharma advisory committees. JK and UC have received research funding from Novartis Pharma. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents, received or pending, or royalties.
Figures

Similar articles
-
Everolimus: a proliferation signal inhibitor with clinical applications in organ transplantation, oncology, and cardiology.Pharmacotherapy. 2010 Oct;30(10):1044-56. doi: 10.1592/phco.30.10.1044. Pharmacotherapy. 2010. PMID: 20874042 Review.
-
Clinical pharmacokinetics of everolimus.Clin Pharmacokinet. 2004;43(2):83-95. doi: 10.2165/00003088-200443020-00002. Clin Pharmacokinet. 2004. PMID: 14748618 Review.
-
Sirolimus and everolimus in kidney transplantation.Drug Discov Today. 2015 Oct;20(10):1243-9. doi: 10.1016/j.drudis.2015.05.006. Epub 2015 Jun 4. Drug Discov Today. 2015. PMID: 26050578 Review.
-
Everolimus: a guide to its use in liver transplantation.BioDrugs. 2013 Aug;27(4):407-11. doi: 10.1007/s40259-013-0041-6. BioDrugs. 2013. PMID: 23696253 Review.
-
mTOR inhibitors in pancreas transplant: adverse effects and drug-drug interactions.Expert Opin Drug Metab Toxicol. 2017 Apr;13(4):367-385. doi: 10.1080/17425255.2017.1239708. Epub 2016 Sep 28. Expert Opin Drug Metab Toxicol. 2017. PMID: 27659512 Review.
Cited by
-
The Newest Generation of Drug-eluting Stents and Beyond.Eur Cardiol. 2018 Aug;13(1):54-59. doi: 10.15420/ecr.2018:8:2. Eur Cardiol. 2018. PMID: 30310472 Free PMC article. Review.
-
Screening the medicine for malaria venture's Pandemic Response Box to identify novel inhibitors of Candida albicans and Candida auris biofilm formation.APMIS. 2023 Nov;131(11):613-625. doi: 10.1111/apm.13342. Epub 2023 Jun 20. APMIS. 2023. PMID: 37337909 Free PMC article.
-
Modulating the PI3K Signalling Pathway in Activated PI3K Delta Syndrome: a Clinical Perspective.J Clin Immunol. 2023 Dec 27;44(1):34. doi: 10.1007/s10875-023-01626-0. J Clin Immunol. 2023. PMID: 38148368 Free PMC article. Review.
-
Lymphangioleiomyomatosis and mTOR inhibitors in real world-a narrative review.J Thorac Dis. 2023 Nov 30;15(11):6333-6344. doi: 10.21037/jtd-23-778. Epub 2023 Nov 16. J Thorac Dis. 2023. PMID: 38090328 Free PMC article. Review.
-
Pharmacology of Aging: Drosophila as a Tool to Validate Drug Targets for Healthy Lifespan.Aging Biol. 2024 Sep 13;2(1):20240034. doi: 10.59368/agingbio.20240034. Aging Biol. 2024. PMID: 39346601 Free PMC article.
References
-
- Peddi VR, Wiseman A, Chavin K, et al. Review of combination therapy with mTOR inhibitors and tacrolimus minimization after transplantation. Transplant Rev. 2013;27(4):97–107. - PubMed
-
- Yost SE, Byrne R, Kaplan B. Transplantation: mTOR inhibition in kidney transplant recipients. Nat Rev Nephrol. 2011;7(10):553–5. - PubMed
-
- Manito N, Delgado JF, Crespo-Leiro MG, et al. Clinical recommendations for the use of everolimus in heart transplantation. Transplant Rev (Orlando) 2010;24(3):129–42. - PubMed
-
- De Simone P, Metselaar HJ, Fischer L, et al. Conversion from a calcineurin inhibitor to everolimus therapy in maintenance liver transplant recipients: a prospective, randomized, multicenter trial. Liver Transplant. 2009;15(10):1262–9. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
