Acrofacial Dysostosis, Cincinnati Type, a Mandibulofacial Dysostosis Syndrome with Limb Anomalies, Is Caused by POLR1A Dysfunction
- PMID: 25913037
- PMCID: PMC4570288
- DOI: 10.1016/j.ajhg.2015.03.011
Acrofacial Dysostosis, Cincinnati Type, a Mandibulofacial Dysostosis Syndrome with Limb Anomalies, Is Caused by POLR1A Dysfunction
Abstract
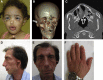
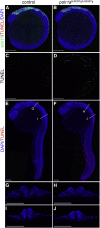
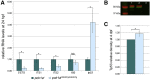
We report three individuals with a cranioskeletal malformation syndrome that we define as acrofacial dysostosis, Cincinnati type. Each individual has a heterozygous mutation in POLR1A, which encodes a core component of RNA polymerase 1. All three individuals exhibit varying degrees of mandibulofacial dysostosis, and two additionally have limb anomalies. Consistent with this observation, we discovered that polr1a mutant zebrafish exhibited cranioskeletal anomalies mimicking the human phenotype. polr1a loss of function led to perturbed ribosome biogenesis and p53-dependent cell death, resulting in a deficiency of neural-crest-derived skeletal precursor cells and consequently craniofacial anomalies. Our findings expand the genotypic and phenotypic heterogeneity of congenital acrofacial disorders caused by disruption of ribosome biogenesis.
Copyright © 2015 The American Society of Human Genetics. Published by Elsevier Inc. All rights reserved.
Figures





References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

