Formation of stable small cell number three-dimensional ovarian cancer spheroids using hanging drop arrays for preclinical drug sensitivity assays
- PMID: 25913133
- PMCID: PMC4480341
- DOI: 10.1016/j.ygyno.2015.04.014
Formation of stable small cell number three-dimensional ovarian cancer spheroids using hanging drop arrays for preclinical drug sensitivity assays
Abstract
Background: Ovarian cancer grows and metastasizes from multicellular spheroidal aggregates within the ascites fluid. Multicellular tumor spheroids are therefore physiologically significant 3D in vitro models for ovarian cancer research. Conventional hanging drop cultures require high starting cell numbers, and are tedious for long-term maintenance. In this study, we generate stable, uniform multicellular spheroids using very small number of ovarian cancer cells in a novel 384 well hanging drop array platform.
Methods: We used novel tumor spheroid platform and two ovarian cancer cell lines (A2780 and OVCAR3) to demonstrate the stable incorporation of as few as 10 cells into a single spheroid.
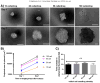
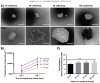
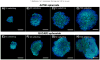
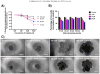
Results: Spheroids had uniform geometry, with projected areas (42.60×10(3)μm-475.22×10(3)μm(2) for A2780 spheroids and 37.24×10(3)μm(2)-281.01×10(3)μm(2) for OVCAR3 spheroids) that varied as a function of the initial cell seeding density. Phalloidin and nuclear stains indicated cells formed tightly packed spheroids with demarcated boundaries and cell-cell interaction within spheroids. Cells within spheroids demonstrated over 85% viability. 3D tumor spheroids demonstrated greater resistance (70-80% viability) to cisplatin chemotherapy compared to 2D cultures (30-50% viability).
Conclusions: Ovarian cancer spheroids can be generated from limited cell numbers in high throughput 384 well plates with high viability. Spheroids demonstrate therapeutic resistance relative to cells in traditional 2D culture. Stable incorporation of low cell numbers is advantageous when translating this research to rare patient-derived cells. This system can be used to understand ovarian cancer spheroid biology, as well as carry out preclinical drug sensitivity assays.
Keywords: 3D culture; High throughput; Multicellular tumor spheroids; Ovarian cancer; Preclinical drug testing.
Copyright © 2015 Elsevier Inc. All rights reserved.
Figures


References
-
- Cannistra SA. Cancer of the ovary. N Engl J Med. 2004;351:2519–29. - PubMed
-
- Lowe KA, Chia VM, Taylor A, et al. An international assessment of ovarian cancer incidence and mortality. Gynecol Oncol. 2013;130:107–14. - PubMed
-
- Permuth-Wey J, Sellers TA. Epidemiology of ovarian cancer. Methods Mol Biol. 2009;472:413–37. - PubMed
-
- Luvero D, Milani A, Ledermann JA. Treatment options in recurrent ovarian cancer: latest evidence and clinical potential. Therapeutic Advances in Medical Oncology. 2014 http://dx.doi.org/10.1177/1758834014544121. - DOI - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

