Neuroendocrine control of the onset of puberty
- PMID: 25913220
- PMCID: PMC4457677
- DOI: 10.1016/j.yfrne.2015.04.002
Neuroendocrine control of the onset of puberty
Abstract
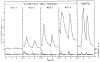
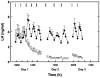
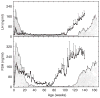
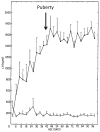
This chapter is based on the Geoffrey Harris Memorial Lecture presented at the 8th International Congress of Neuroendocrinology, which was held in Sydney, August 2014. It provides the development of our understanding of the neuroendocrine control of puberty since Harris proposed in his 1955 monograph (Harris, 1955) that "a major factor responsible for puberty is an increased rate of release of pituitary gonadotrophin" and posited "that a neural (hypothalamic) stimulus, via the hypophysial portal vessels, may be involved." Emphasis is placed on the neurobiological mechanisms governing puberty in highly evolved primates, although an attempt is made to reverse translate a model for the timing of puberty in man and monkey to non-primate species.
Keywords: GnRH pulse generation; GnRH surge generation; Human; Kisspeptin; Puberty; Rat; Rhesus monkey; Sheep.
Copyright © 2015 Elsevier Inc. All rights reserved.
Figures

References
-
- Gove PB. Webster’s Third New International Dictionary of the English Language Unabridged. G & C Merriam; Springfield, MA: 1961.
-
- Harris GW. Neural control of the Pituitary Gland. Edward Arnold; London: 1955.
-
- Matsuo H, Baba Y, Nair RM, Arimura A, Schally AV. Structure of the porcine LH- and FSH-releasing hormone. I. The proposed amino acid sequence. Biochemical and biophysical research communications. 1971;43:1334–9. - PubMed
-
- Amoss M, Burgus R, Blackwell R, Vale W, Fellows R, Guillemin R. Purification, amino acid composition and N-terminus of the hypothalamic luteinizing hormone releasing factor (LRF) of ovine origin. Biochemical and biophysical research communications. 1971;44:205–10. - PubMed
-
- Dierschke DJ, Bhattacharya AN, Atkinson LE, Knobil E. Circhoral oscillations of plasma LH levels in the ovariectomized rhesus monkey. Endocrinology. 1970;87:850–3. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

