Molecular signatures of major depression
- PMID: 25913401
- PMCID: PMC4425463
- DOI: 10.1016/j.cub.2015.03.008
Molecular signatures of major depression
Abstract
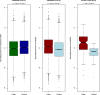
Adversity, particularly in early life, can cause illness. Clues to the responsible mechanisms may lie with the discovery of molecular signatures of stress, some of which include alterations to an individual's somatic genome. Here, using genome sequences from 11,670 women, we observed a highly significant association between a stress-related disease, major depression, and the amount of mtDNA (p = 9.00 × 10(-42), odds ratio 1.33 [95% confidence interval [CI] = 1.29-1.37]) and telomere length (p = 2.84 × 10(-14), odds ratio 0.85 [95% CI = 0.81-0.89]). While both telomere length and mtDNA amount were associated with adverse life events, conditional regression analyses showed the molecular changes were contingent on the depressed state. We tested this hypothesis with experiments in mice, demonstrating that stress causes both molecular changes, which are partly reversible and can be elicited by the administration of corticosterone. Together, these results demonstrate that changes in the amount of mtDNA and telomere length are consequences of stress and entering a depressed state. These findings identify increased amounts of mtDNA as a molecular marker of MD and have important implications for understanding how stress causes the disease.
Copyright © 2015 The Authors. Published by Elsevier Ltd.. All rights reserved.
Figures




Comment in
-
Genetics: dynamic cellular aging markers associated with major depression.Curr Biol. 2015 May 18;25(10):R409-11. doi: 10.1016/j.cub.2015.03.036. Curr Biol. 2015. PMID: 25989078
References
-
- Nanni V., Uher R., Danese A. Childhood maltreatment predicts unfavorable course of illness and treatment outcome in depression: a meta-analysis. Am. J. Psychiatry. 2012;169:141–151. - PubMed
-
- Dong M., Giles W.H., Felitti V.J., Dube S.R., Williams J.E., Chapman D.P., Anda R.F. Insights into causal pathways for ischemic heart disease: adverse childhood experiences study. Circulation. 2004;110:1761–1766. - PubMed
-
- Shonkoff J.P., Garner A.S., Committee on Psychosocial Aspects of Child and Family Health. Committee on Early Childhood, Adoption, and Dependent Care. Section on Developmental and Behavioral Pediatrics The lifelong effects of early childhood adversity and toxic stress. Pediatrics. 2012;129:e232–e246. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

