Xyloketal B suppresses glioblastoma cell proliferation and migration in vitro through inhibiting TRPM7-regulated PI3K/Akt and MEK/ERK signaling pathways
- PMID: 25913706
- PMCID: PMC4413223
- DOI: 10.3390/md13042505
Xyloketal B suppresses glioblastoma cell proliferation and migration in vitro through inhibiting TRPM7-regulated PI3K/Akt and MEK/ERK signaling pathways
Abstract
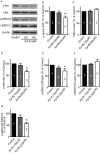
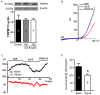
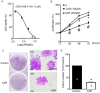
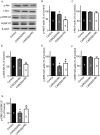
Glioblastoma, the most common and aggressive type of brain tumors, has devastatingly proliferative and invasive characteristics. The need for finding a novel and specific drug target is urgent as the current approaches have limited therapeutic effects in treating glioblastoma. Xyloketal B is a marine compound obtained from mangrove fungus Xylaria sp. (No. 2508) from the South China Sea, and has displayed antioxidant activity and protective effects on endothelial and neuronal oxidative injuries. In this study, we used a glioblastoma U251 cell line to (1) explore the effects of xyloketal B on cell viability, proliferation, and migration; and (2) investigate the underlying molecular mechanisms and signaling pathways. MTT assay, colony formation, wound healing, western blot, and patch clamp techniques were employed. We found that xyloketal B reduced cell viability, proliferation, and migration of U251 cells. In addition, xyloketal B decreased p-Akt and p-ERK1/2 protein expressions. Furthermore, xyloketal B blocked TRPM7 currents in HEK-293 cells overexpressing TRPM7. These effects were confirmed by using a TRPM7 inhibitor, carvacrol, in a parallel experiment. Our findings indicate that TRPM7-regulated PI3K/Akt and MEK/ERK signaling is involved in anti-proliferation and migration effects of xyloketal B on U251 cells, providing in vitro evidence for the marine compound xyloketal B to be a potential drug for treating glioblastoma.
Figures




References
-
- Stupp R., Hegi M.E., Mason W.P., van den Bent M.J., Taphoorn M.J., Janzer R.C., Ludwin S.K., Allgeier A., Fisher B., Belanger K., et al. Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase iii study: 5-Year analysis of the eortc-ncic trial. Lancet Oncol. 2009;10:459–466. doi: 10.1016/S1470-2045(09)70025-7. - DOI - PubMed
-
- Fleming T.P., Saxena A., Clark W.C., Robertson J.T., Oldfield E.H., Aaronson S.A., Ali I.U. Amplification and/or overexpression of platelet-derived growth factor receptors and epidermal growth factor receptor in human glial tumors. Cancer Res. 1992;52:4550–4553. - PubMed
-
- Pelloski C.E., Lin E., Zhang L., Yung W.K., Colman H., Liu J.L., Woo S.Y., Heimberger A.B., Suki D., Prados M., et al. Prognostic associations of activated mitogen-activated protein kinase and akt pathways in glioblastoma. Clin. Cancer Res. 2006;12:3935–3941. doi: 10.1158/1078-0432.CCR-05-2202. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

