Strategies to guide the antibody affinity maturation process
- PMID: 25913818
- PMCID: PMC4456294
- DOI: 10.1016/j.coviro.2015.04.002
Strategies to guide the antibody affinity maturation process
Abstract
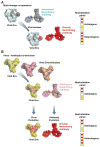
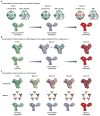
Antibodies with protective activity are critical for vaccine efficacy. Affinity maturation increases antibody activity through multiple rounds of somatic hypermutation and selection in the germinal center. Identification of HIV-1 specific and influenza-specific antibody developmental pathways, as well as characterization of B cell and virus co-evolution in patients, has informed our understanding of antibody development. In order to counteract HIV-1 and influenza viral diversity, broadly neutralizing antibodies precisely target specific sites of vulnerability and require high levels of affinity maturation. We present immunization strategies that attempt to recapitulate these natural processes and guide the affinity maturation process.
Published by Elsevier B.V.
Figures

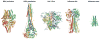
Similar articles
-
Structural and genetic basis for development of broadly neutralizing influenza antibodies.Nature. 2012 Sep 27;489(7417):566-70. doi: 10.1038/nature11371. Epub 2012 Aug 29. Nature. 2012. PMID: 22932267 Free PMC article.
-
What Are the Primary Limitations in B-Cell Affinity Maturation, and How Much Affinity Maturation Can We Drive with Vaccination? Lessons from the Antibody Response to HIV-1.Cold Spring Harb Perspect Biol. 2018 May 1;10(5):a029389. doi: 10.1101/cshperspect.a029389. Cold Spring Harb Perspect Biol. 2018. PMID: 28630079 Free PMC article. Review.
-
Broadly Reactive Influenza Antibodies Are Not Limited by Germinal Center Competition with High-Affinity Antibodies.mBio. 2020 Nov 3;11(6):e01859-20. doi: 10.1128/mBio.01859-20. mBio. 2020. PMID: 33144374 Free PMC article.
-
Translating antibody insights.Science. 2013 Sep 13;341(6151):1151. doi: 10.1126/science.1245018. Science. 2013. PMID: 24030986 No abstract available.
-
Broadly neutralizing antibodies present new prospects to counter highly antigenically diverse viruses.Science. 2012 Jul 13;337(6091):183-6. doi: 10.1126/science.1225416. Science. 2012. PMID: 22798606 Free PMC article. Review.
Cited by
-
BNT162b2 Vaccination after SARS-CoV-2 Infection Changes the Dynamics of Total and Neutralizing Antibodies against SARS-CoV-2: A 6-Month Prospective Cohort Study.Vaccines (Basel). 2023 Jun 20;11(6):1127. doi: 10.3390/vaccines11061127. Vaccines (Basel). 2023. PMID: 37376516 Free PMC article.
-
Deconstructing the Antiviral Neutralizing-Antibody Response: Implications for Vaccine Development and Immunity.Microbiol Mol Biol Rev. 2016 Oct 26;80(4):989-1010. doi: 10.1128/MMBR.00024-15. Print 2016 Dec. Microbiol Mol Biol Rev. 2016. PMID: 27784796 Free PMC article. Review.
-
Designs of Antigen Structure and Composition for Improved Protein-Based Vaccine Efficacy.Front Immunol. 2020 Feb 24;11:283. doi: 10.3389/fimmu.2020.00283. eCollection 2020. Front Immunol. 2020. PMID: 32153587 Free PMC article. Review.
-
New Member of the V1V2-Directed CAP256-VRC26 Lineage That Shows Increased Breadth and Exceptional Potency.J Virol. 2015 Oct 14;90(1):76-91. doi: 10.1128/JVI.01791-15. Print 2016 Jan 1. J Virol. 2015. PMID: 26468542 Free PMC article.
-
Neutralizing the threat: harnessing broadly neutralizing antibodies against HIV-1 for treatment and prevention.Microb Cell. 2024 Jul 3;11:207-220. doi: 10.15698/mic2024.07.826. eCollection 2024. Microb Cell. 2024. PMID: 38975023 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

