Sensory Cortical Control of a Visually Induced Arrest Behavior via Corticotectal Projections
- PMID: 25913860
- PMCID: PMC4452020
- DOI: 10.1016/j.neuron.2015.03.048
Sensory Cortical Control of a Visually Induced Arrest Behavior via Corticotectal Projections
Abstract
Innate defense behaviors (IDBs) evoked by threatening sensory stimuli are essential for animal survival. Although subcortical circuits are implicated in IDBs, it remains largely unclear whether sensory cortex modulates IDBs and what the underlying neural pathways are. Here, we show that optogenetic silencing of corticotectal projections from layer 5 (L5) of the mouse primary visual cortex (V1) to the superior colliculus (SC) significantly reduces an SC-dependent innate behavior (i.e., temporary suspension of locomotion upon a sudden flash of light as short as milliseconds). Surprisingly, optogenetic activation of SC-projecting neurons in V1 or their axon terminals in SC sufficiently elicits the behavior, in contrast to other major L5 corticofugal projections. Thus, via the same corticofugal projection, visual cortex not only modulates the light-induced arrest behavior, but also can directly drive the behavior. Our results suggest that sensory cortex may play a previously unrecognized role in the top-down initiation of sensory-motor behaviors.
Copyright © 2015 Elsevier Inc. All rights reserved.
Figures


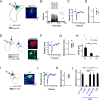
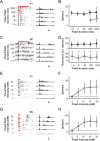


References
-
- Bordi F, LeDoux J. Sensory tuning beyond the sensory system: an initial analysis of auditory response properties of neurons in the lateral amygdaloid nucleus and overlying areas of the striatum. The Journal of neuroscience: the official journal of the Society for Neuroscience. 1992;12:2493–2503. - PMC - PubMed
-
- Brandao ML, Borelli KG, Nobre MJ, Santos JM, Albrechet-Souza L, Oliveira AR, Martinez RC. Gabaergic regulation of the neural organization of fear in the midbrain tectum. Neuroscience and biobehavioral reviews. 2005;29:1299–1311. - PubMed
-
- Ciaramitaro VM, Todd WE, Rosenquist AC. Disinhibition of the superior colliculus restores orienting to visual stimuli in the hemianopic field of the cat. The Journal of comparative neurology. 1997;387:568–587. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

