N-terminal modifications of cellular proteins: The enzymes involved, their substrate specificities and biological effects
- PMID: 25914051
- PMCID: PMC4692089
- DOI: 10.1002/pmic.201400619
N-terminal modifications of cellular proteins: The enzymes involved, their substrate specificities and biological effects
Abstract
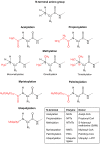
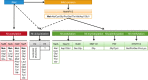
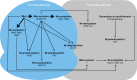
The vast majority of eukaryotic proteins are N-terminally modified by one or more processing enzymes. Enzymes acting on the very first amino acid of a polypeptide include different peptidases, transferases, and ligases. Methionine aminopeptidases excise the initiator methionine leaving the nascent polypeptide with a newly exposed amino acid that may be further modified. N-terminal acetyl-, methyl-, myristoyl-, and palmitoyltransferases may attach an acetyl, methyl, myristoyl, or palmitoyl group, respectively, to the α-amino group of the target protein N-terminus. With the action of ubiquitin ligases, one or several ubiquitin molecules are transferred, and hence, constitute the N-terminal modification. Modifications at protein N-termini represent an important contribution to proteomic diversity and complexity, and are essential for protein regulation and cellular signaling. Consequently, dysregulation of the N-terminal modifying enzymes is implicated in human diseases. We here review the different protein N-terminal modifications occurring co- or post-translationally with emphasis on the responsible enzymes and their substrate specificities.
Keywords: Acetylation; Cell biology; N-terminal; Protein modification; Substrate specificity; α-amino group.
© 2015 The Authors. PROTEOMICS Published by Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim.
Figures
References
-
- Sherman F, Stewart JW, Tsunasawa S. Methionine or not methionine at the beginning of a protein. Bioessays. 1985;3:27–31. - PubMed
-
- Starheim KK, Gevaert K, Arnesen T. Protein N-terminal acetyltransferases: when the start matters. Trends Biochem. Sci. 2012;37:152–161. - PubMed
-
- Martin DD, Beauchamp E, Berthiaume LG. Post-translational myristoylation: Fat matters in cellular life and death. Biochimie. 2011;93:18–31. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

