An Interdomain KCNH2 Mutation Produces an Intermediate Long QT Syndrome
- PMID: 25914329
- PMCID: PMC4667707
- DOI: 10.1002/humu.22805
An Interdomain KCNH2 Mutation Produces an Intermediate Long QT Syndrome
Erratum in
-
Corrigendum.Hum Mutat. 2019 Mar;40(3):357. doi: 10.1002/humu.23711. Hum Mutat. 2019. PMID: 30740826 No abstract available.
Abstract
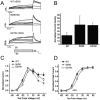
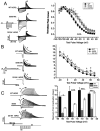
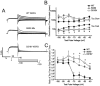
Hereditary long QT syndrome is caused by deleterious mutation in one of several genetic loci, including locus LQT2 that contains the KCNH2 gene (or hERG, human ether-a-go-go related gene), causing faulty cardiac repolarization. Here, we describe and characterize a novel mutation, p.Asp219Val in the hERG channel, identified in an 11-year-old male with syncope and prolonged QT interval. Genetic sequencing showed a nonsynonymous variation in KCNH2 (c.656A>T: amino acid p.Asp219Val). p.Asp219Val resides in a region of the channel predicted to be unstructured and flexible, located between the PAS (Per-Arnt-Sim) domain and its interaction sites in the transmembrane domain. The p.Asp219Val hERG channel produced K(+) current that activated with modest changes in voltage dependence. Mutant channels were also slower to inactivate, recovered from inactivation more readily and demonstrated a significantly accelerated deactivation rate compared with the slow deactivation of wild-type channels. The intermediate nature of the biophysical perturbation is consistent with the degree of severity in the clinical phenotype. The findings of this study demonstrate a previously unknown role of the proximal N-terminus in deactivation and support the hypothesis that the proximal N-terminal domain is essential in maintaining slow hERG deactivation.
Keywords: KCNH2; LQT2; deactivation; hERG; ventricular arrhythmia.
© 2015 WILEY PERIODICALS, INC.
Conflict of interest statement
The authors have no conflicts of interest.
Figures



References
-
- Anderson CL, Delisle BP, Anson BD, Kilby JA, Will ML, Tester DJ, Gong Q, Zhou Z, Ackerman MJ, January CT. Most LQT2 mutations reduce Kv11.1 (hERG) current by a class 2 (trafficking-deficient) mechanism. Circulation. 2006;113:365–373. - PubMed
-
- Bian J, Cui J, McDonald TV. HERG K(+) channel activity is regulated by changes in phosphatidyl inositol 4,5-bisphosphate. Circulation research. 2001;89:1168–1176. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

