Managing refractory Crohn's disease: challenges and solutions
- PMID: 25914555
- PMCID: PMC4401331
- DOI: 10.2147/CEG.S61868
Managing refractory Crohn's disease: challenges and solutions
Abstract
The goals of treatment for active Crohn's disease (CD) are to achieve clinical remission and improve quality of life. Conventional therapeutics for moderate-to-severe CD include 5-aminosalicylic acid, corticosteroids, purine analogs, azathioprine, and 6-mercaptopurine. Patients who fail to respond to conventional therapy are treated with tumor necrosis factor (TNF)-α inhibitors such as infliximab and adalimumab, but their efficacy is limited due to primary nonresponse or loss of response. It is suggested that this requires switch to another TNF-α inhibitor, a combination therapy with TNF-α blockade plus azathioprine, or granulocyte and monocyte adsorptive apheresis, and that other therapeutic options having different mechanisms of action, such as blockade of inflammatory cytokines or adhesion molecules, are needed. Natalizumab and vedolizumab are neutralizing antibodies directed against integrin α4 and α4β7, respectively. Ustekinumab is a neutralizing antibody directed against the receptors for interleukin-12 and interleukin-23. Here, we provide an overview of therapeutic treatments that are effective and currently available for CD patients, as well as some that likely will be available in the near future. We also discuss the advantages of managing patients with refractory CD using a combination of TNF-α inhibitors plus azathioprine or intensive monocyte adsorptive apheresis.
Keywords: adalimumab; combination therapy; complete remission; granulocyte and monocyte adsorptive apheresis.
Figures
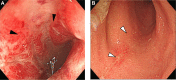
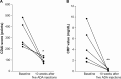
References
-
- Hanauer SB, Feagan BG, Lichtenstein GR, et al. Maintenance infliximab for Crohn’s disease: the ACCENT I randomised trial. Lancet. 2002;359(9317):1541–1549. - PubMed
-
- Hanauer SB, Sandborn WJ, Rutgeerts P, et al. Human anti-tumor necrosis factor monoclonal antibody (adalimumab) in Crohn’s disease: the CLASSIC-I trial. Gastroenterology. 2006;130(2):323–333. - PubMed
-
- Colombel JF, Sandborn WJ, Rutgeerts P, et al. Adalimumab for maintenance of clinical response and remission in patients with Crohn’s disease: the CHARM trial. Gastroenterology. 2007;132(1):52–65. - PubMed
Publication types
LinkOut - more resources
Full Text Sources

