Characterization of optic nerve regeneration using transgenic zebrafish
- PMID: 25914619
- PMCID: PMC4391235
- DOI: 10.3389/fncel.2015.00118
Characterization of optic nerve regeneration using transgenic zebrafish
Abstract
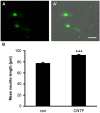
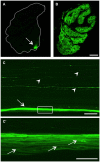
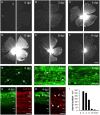
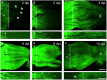
In contrast to the adult mammalian central nervous system (CNS), fish are able to functionally regenerate severed axons upon injury. Although the zebrafish is a well-established model vertebrate for genetic and developmental studies, its use for anatomical studies of axon regeneration has been hampered by the paucity of appropriate tools to visualize re-growing axons in the adult CNS. On this account, we used transgenic zebrafish that express enhanced green fluorescent protein (GFP) under the control of a GAP-43 promoter. In adult, naïve retinae, GFP was restricted to young retinal ganglion cells (RGCs) and their axons. Within the optic nerve, these fluorescent axons congregated in a distinct strand at the nerve periphery, indicating age-related order. Upon optic nerve crush, GFP expression was markedly induced in RGC somata and intra-retinal axons at 4 to at least 14 days post injury. Moreover, individual axons were visualized in their natural environment of the optic nerve using wholemount tissue clearing and confocal microscopy. With this novel approach, regenerating axons were clearly detectable beyond the injury site as early as 2 days after injury and grew past the optic chiasm by 4 days. Regenerating axons in the entire optic nerve were labeled from 6 to at least 14 days after injury, thereby allowing detailed visualization of the complete regeneration process. Therefore, this new approach could now be used in combination with expression knockdown or pharmacological manipulations to analyze the relevance of specific proteins and signaling cascades for axonal regeneration in vivo. In addition, the RGC-specific GFP expression facilitated accurate evaluation of neurite growth in dissociated retinal cultures. This fast in vitro assay now enables the screening of compound and expression libraries. Overall, the presented methodologies provide exciting possibilities to investigate the molecular mechanisms underlying successful CNS regeneration in zebrafish.
Keywords: GFP; axon regeneration; optic nerve regeneration; tissue clearing; transgenic zebrafish.
Figures


References
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

