Identification of the antiepileptic racetam binding site in the synaptic vesicle protein 2A by molecular dynamics and docking simulations
- PMID: 25914622
- PMCID: PMC4392693
- DOI: 10.3389/fncel.2015.00125
Identification of the antiepileptic racetam binding site in the synaptic vesicle protein 2A by molecular dynamics and docking simulations
Abstract
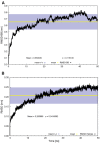
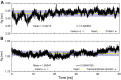
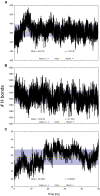
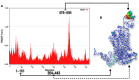
Synaptic vesicle protein 2A (SV2A) is an integral membrane protein necessary for the proper function of the central nervous system and is associated to the physiopathology of epilepsy. SV2A is the molecular target of the anti-epileptic drug levetiracetam and its racetam analogs. The racetam binding site in SV2A and the non-covalent interactions between racetams and SV2A are currently unknown; therefore, an in silico study was performed to explore these issues. Since SV2A has not been structurally characterized with X-ray crystallography or nuclear magnetic resonance, a three-dimensional (3D) model was built. The model was refined by performing a molecular dynamics simulation (MDS) and the interactions of SV2A with the racetams were determined by docking studies. A reliable 3D model of SV2A was obtained; it reached structural equilibrium during the last 15 ns of the MDS (50 ns) with remaining structural motions in the N-terminus and long cytoplasmic loop. The docking studies revealed that hydrophobic interactions and hydrogen bonds participate importantly in ligand recognition within the binding site. Residues T456, S665, W666, D670 and L689 were important for racetam binding within the trans-membrane hydrophilic core of SV2A. Identifying the racetam binding site within SV2A should facilitate the synthesis of suitable radio-ligands to study treatment response and possibly epilepsy progression.
Keywords: SV2A; brivaracetam; epilepsy; levetiracetam; seletracetam.
Figures



Similar articles
-
Combining modelling and mutagenesis studies of synaptic vesicle protein 2A to identify a series of residues involved in racetam binding.Biochem Soc Trans. 2011 Oct;39(5):1341-7. doi: 10.1042/BST0391341. Biochem Soc Trans. 2011. PMID: 21936812
-
Binding characteristics of brivaracetam, a selective, high affinity SV2A ligand in rat, mouse and human brain: relationship to anti-convulsant properties.Eur J Pharmacol. 2011 Aug 16;664(1-3):36-44. doi: 10.1016/j.ejphar.2011.04.064. Epub 2011 May 8. Eur J Pharmacol. 2011. PMID: 21575627
-
Exploring the interaction of SV2A with racetams using homology modelling, molecular dynamics and site-directed mutagenesis.PLoS One. 2015 Feb 18;10(2):e0116589. doi: 10.1371/journal.pone.0116589. eCollection 2015. PLoS One. 2015. PMID: 25692762 Free PMC article.
-
Brivaracetam: Rationale for discovery and preclinical profile of a selective SV2A ligand for epilepsy treatment.Epilepsia. 2016 Apr;57(4):538-48. doi: 10.1111/epi.13340. Epub 2016 Feb 26. Epilepsia. 2016. PMID: 26920914 Review.
-
Therapeutic Role of Synaptic Vesicle Glycoprotein 2A (SV2A) in Modulating Epileptogenesis.CNS Neurol Disord Drug Targets. 2017;16(4):463-471. doi: 10.2174/1871527316666170404115027. CNS Neurol Disord Drug Targets. 2017. PMID: 28393712 Review.
Cited by
-
The iTRAPs: Guardians of Synaptic Vesicle Cargo Retrieval During Endocytosis.Front Synaptic Neurosci. 2016 Feb 9;8:1. doi: 10.3389/fnsyn.2016.00001. eCollection 2016. Front Synaptic Neurosci. 2016. PMID: 26903854 Free PMC article. Review.
-
Synaptic Vesicle Protein 2A Expression in Glutamatergic Terminals Is Associated with the Response to Levetiracetam Treatment.Brain Sci. 2021 Apr 23;11(5):531. doi: 10.3390/brainsci11050531. Brain Sci. 2021. PMID: 33922424 Free PMC article.
-
Synaptic vesicle protein 2: A multi-faceted regulator of secretion.Semin Cell Dev Biol. 2019 Nov;95:130-141. doi: 10.1016/j.semcdb.2019.02.003. Epub 2019 Mar 21. Semin Cell Dev Biol. 2019. PMID: 30826548 Free PMC article. Review.
-
Imaging Synaptic Density: The Next Holy Grail of Neuroscience?Front Neurosci. 2022 Mar 25;16:796129. doi: 10.3389/fnins.2022.796129. eCollection 2022. Front Neurosci. 2022. PMID: 35401097 Free PMC article. Review.
-
Control of Synaptotagmin-1 Trafficking by SV2A-Mechanism and Consequences for Presynaptic Function and Dysfunction.J Neurochem. 2025 Jan;169(1):e16308. doi: 10.1111/jnc.16308. J Neurochem. 2025. PMID: 39853744 Free PMC article. Review.
References
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

