Comparison of routes for achieving parenteral access with a focus on the management of patients with Ebola virus disease
- PMID: 25914907
- PMCID: PMC4455225
- DOI: 10.1002/14651858.CD011386.pub2
Comparison of routes for achieving parenteral access with a focus on the management of patients with Ebola virus disease
Abstract
Background: Dehydration is an important cause of death in patients with Ebola virus disease (EVD). Parenteral fluids are often required in patients with fluid requirements in excess of their oral intake. The peripheral intravenous route is the most commonly used method of parenteral access, but inserting and maintaining an intravenous line can be challenging in the context of EVD. Therefore it is important to consider the advantages and disadvantages of different routes for achieving parenteral access (e.g. intravenous, intraosseous, subcutaneous and intraperitoneal).
Objectives: To compare the reliability, ease of use and speed of insertion of different parenteral access methods.
Search methods: We ran the search on 17 November 2014. We searched the Cochrane Injuries Group's Specialised Register, Cochrane Central Register of Controlled Trials (CENTRAL, The Cochrane Library), Ovid MEDLINE(R) In-Process & Other Non-Indexed Citations, Ovid MEDLINE(R) Daily, Ovid MEDLINE(R) and Ovid OLDMEDLINE(R), Embase Classic + Embase (OvidSP), CINAHL (EBSCOhost), clinicaltrials.gov and screened reference lists.
Selection criteria: Randomised controlled trials comparing different parenteral routes for the infusion of fluids or medication.
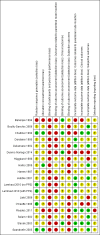
Data collection and analysis: Two review authors examined the titles and abstracts of records obtained by searching the electronic databases to determine eligibility. Two review authors extracted data from the included trials and assessed the risk of bias. Outcome measures of interest were success of insertion; time required for insertion; number of insertion attempts; number of dislodgements; time period with functional access; local site reactions; clinicians' perception of ease of administration; needlestick injury to healthcare workers; patients' discomfort; and mortality. For trials involving the administration of fluids we also collected data on the volume of fluid infused, changes in serum electrolytes and markers of renal function. We rated the quality of the evidence as 'high', 'moderate', 'low' or 'very low' according to the GRADE approach for the following outcomes: success of insertion, time required for insertion, number of dislodgements, volume of fluid infused and needlestick injuries.
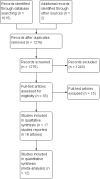
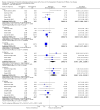
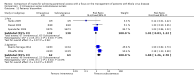
Main results: We included 17 trials involving 885 participants. Parenteral access was used to infuse fluids in 11 trials and medications in six trials. None of the trials involved patients with EVD. Intravenous and intraosseous access was compared in four trials; intravenous and subcutaneous access in 11; peripheral intravenous and intraperitoneal access in one; saphenous vein cutdown and intraosseous access in one; and intraperitoneal with subcutaneous access in one. All of the trials assessing the intravenous method involved peripheral intravenous access.We judged few trials to be at low risk of bias for any of the assessed domains.Compared to the intraosseous group, patients in the intravenous group were more likely to experience an insertion failure (risk ratio (RR) 3.89, 95% confidence interval (CI) 2.39 to 6.33; n = 242; GRADE rating: low). We did not pool data for time to insertion but estimates from the trials suggest that inserting intravenous access takes longer (GRADE rating: moderate). Clinicians judged the intravenous route to be easier to insert (RR 0.15, 95% CI 0.04 to 0.61; n = 182). A larger volume of fluids was infused via the intravenous route (GRADE rating: moderate). There was no evidence of a difference between the two routes for any other outcomes, including adverse events.Compared to the subcutaneous group, patients in the intravenous group were more likely to experience an insertion failure (RR 14.79, 95% CI 2.87 to 76.08; n = 238; GRADE rating: moderate) and dislodgement of the device (RR 3.78, 95% CI 1.16 to 12.34; n = 67; GRADE rating: low). Clinicians also judged the intravenous route as being more difficult to insert and patients were more likely to be agitated in the intravenous group. Patients in the intravenous group were more likely to develop a local infection and phlebitis, but were less likely to develop erythema, oedema or swelling than those in the subcutaneous group. A larger volume of fluids was infused into patients via the intravenous route. There was no evidence of a difference between the two routes for any other outcome.There were insufficient data to reliably determine if the risk of insertion failure differed between the saphenous vein cutdown (SVC) and intraosseous method (RR 4.00, 95% CI 0.51 to 31.13; GRADE rating: low). Insertion using SVC took longer than the intraosseous method (MD 219.60 seconds, 95% CI 135.44 to 303.76; GRADE rating: moderate). There were no data and therefore there was no evidence of a difference between the two routes for any other outcome.There were insufficient data to reliably determine the relative effects of intraperitoneal or central intravenous access relative to any other parenteral access method.
Authors' conclusions: There are several different ways of achieving parenteral access in patients who are unable meet their fluid requirements with oral intake alone. The quality of the evidence, as assessed using the GRADE criteria, is somewhat limited because of the lack of adequately powered trials at low risk of bias. However, we believe that there is sufficient evidence to draw the following conclusions: if peripheral intravenous access can be achieved easily, this allows infusion of larger volumes of fluid than other routes; but if this is not possible, the intraosseous and subcutaneous routes are viable alternatives. The subcutaneous route may be suitable for patients who are not severely dehydrated but in whom ongoing fluid losses cannot be met by oral intake.A film to accompany this review can be viewed here (http://youtu.be/ArVPzkf93ng).
Conflict of interest statement
KK, GT, DB and HS have no known conflicts of interest.
AP: My institution receives financial support for trials from Fresenius Kabi and CSL Behring.
TH: My institution receives financial support for trials from Fresenius Kabi and CSL Behring.
IR: We (the Cochrane Injuries Group) received a small grant from The Cochrane Collaboration to complete this Cochrane Review.
Figures





























Update of
References
References to studies included in this review
Banerjee 1994 {published data only}
-
- Banerjee S, Singhi S, Singh SC, Singh M. The intraosseous route is a suitable alternative to intravenous route for fluid resuscitation in severely dehydrated children. Indian Pediatrics 1994;31(12):1511‐20. - PubMed
Boullu‐Sanchis 2006 {published data only}
-
- Boullu‐Sanchis S, Ortega F, Chabrier G, Busch MS, Uhl C, Pinget M, et al. Efficacy of short term continuous subcutaneous insulin lispro versus continuous intravenous regular insulin in poorly controlled, hospitalized, type 2 diabetic patients. Diabetes & Metabolism 2006;32(4):350‐7. - PubMed
Challiner 1994 {published data only}
Dardaine 1995 {published data only}
-
- Dardaine V, Garrigue MA, Rapin CH, Constans T. Metabolic and hormonal changes induced by hypodermoclysis of glucose‐saline solution in elderly patients. Journals of Gerontology Series A‐Biological Sciences & Medical Sciences 1995;50(6):M334‐6. - PubMed
Delamaire 1992 {published data only}
-
- Delamaire D, Meigne B, Lecoq A, Cattenoz C, Michel M, Miche O. A rehydration randomized trial favoring the subcutaneous route in the elderly [Un essai controle d'hydratation en faveur d'une actualisation de l'hypodermoclyse chez le sujet age]. Revue de Medecine Interne 1992;13(7):S321.
Duems Noriega 2014 {published data only}
-
- Duems Noriega O, Arino Blasco S. Efficacy of the subcutaneous route compared to intravenous hydration in the elderly hospitalised patient: a randomised controlled study. Revista Espanola de Geriatria y Gerontologia 2014;49(3):103‐7. - PubMed
Hägglund 1998 {published data only}
-
- Hägglund H, Ringdén O, Agren B, Wennberg L, Remberger M, Rundquist L, et al. Intraosseous compared to intravenous infusion of allogeneic bone marrow. Bone Marrow Transplantation 1998;21(4):331‐5. - PubMed
Harbo 2009 {published data only}
-
- Harbo T, Andersen H, Hess A, Hansen K, Sindrup SH, Jakobsen J. Subcutaneous versus intravenous immunoglobulin in multifocal motor neuropathy: a randomized, single‐blinded cross‐over trial. European Journal of Neurology 2009;16(5):631‐8. - PubMed
Harvey 1987 {published data only}
-
- Harvey VJ, Slevin ML, Aherne GW, Littleton P, Johnston A, Wrigley PF. Subcutaneous infusion of bleomycin‐‐a practical alternative to intravenous infusion. Journal of Clinical Oncology 1987;5(4):648‐50. - PubMed
Hubble 2001 {published data only}
-
- Hubble MW, Trigg DC. Training prehospital personnel in saphenous vein cutdown and adult intraosseous access techniques. Prehospital Emergency Care 2001;5(2):181‐9. - PubMed
Lamhaut 2010 (no PPE) {published data only}
-
- Lamhaut L, Dagron C, Apriotesei R, Gouvernaire J, Elie C, Marx JS, et al. Comparison of intravenous and intraosseous access by pre‐hospital medical emergency personnel with and without CBRN protective equipment. Resuscitation 2010;81(1):65‐8. - PubMed
Lamhaut 2010 (with PPE) {published data only}
-
- Lamhaut L, Dagron C, Apriotesei R, Gouvernaire J, Elie C, Marx JS, et al. Comparison of intravenous and intraosseous access by pre‐hospital medical emergency personnel with and without CBRN protective equipment. Resuscitation 2010;81(1):65‐8. - PubMed
Liebl 2009 {published data only}
-
- Liebl A, Hoogma R, Renard E, Geelhoed‐Duijvestijn PH, Klein E, Diglas J, et al. A reduction in severe hypoglycaemia in type 1 diabetes in a randomized crossover study of continuous intraperitoneal compared with subcutaneous insulin infusion. Diabetes, Obesity & Metabolism 2009;11(11):1001‐8. - PubMed
O'Keeffe 1996 {published data only}
-
- O'Keeffe ST, Lavan JN. Subcutaneous fluids in elderly hospital patients with cognitive impairment. Gerontology 1996;42(1):36‐9. - PubMed
Reades 2011 {published data only}
-
- Reades R, Studnek JR, Vandeventer S, Garrett J. Intraosseous versus intravenous vascular access during out‐of‐hospital cardiac arrest: a randomized controlled trial. Annals of Emergency Medicine 2011;58(6):509‐16. - PubMed
Selam 1983 {published data only}
-
- Selam JL, Slingeneyer A, Hedon B, Mares P, Beraud JJ, Mirouze J. Long‐term ambulatory peritoneal insulin infusion of brittle diabetes with portable pumps: comparison with intravenous and subcutaneous routes. Diabetes Care 1983;6(2):105‐11. - PubMed
Slesak 2003 {published data only}
-
- Slesak G, Schnurle JW, Kinzel E, Jakob J, Dietz PK. Comparison of subcutaneous and intravenous rehydration in geriatric patients: a randomized trial. Journal of the American Geriatrics Society 2003;51(2):155‐60. - PubMed
Spandorfer 2005 {published data only}
-
- Spandorfer PR, Alessandrini EA, Joffe MD, Localio R, Shaw KN. Oral versus intravenous rehydration of moderately dehydrated children: a randomized, controlled trial. Pediatrics 2005;115:295‐301. - PubMed
References to studies excluded from this review
Chavez‐Negrete 1991 {published data only}
-
- Chavez‐Negrete A, Majluf Cruz S, Frati Munari A, Perches A, Arguero R. Treatment of hemorrhagic shock with intraosseus or intravenous infusion of hypertonic saline dextran solution. European Surgical Research 1991;23(2):123‐9. - PubMed
Ismael 2012 {published data only}
-
- Ismael G, Hegg R, Muehlbauer S, Heinzmann D, Lum B, Kim S, et al. Subcutaneous versus intravenous administration of (neo)adjuvant trastuzumab in patients with HER2‐positive, clinical stage I‐III breast cancer (HannaH study): a phase 3, open‐label, multicentre, randomised trial. Lancet Oncology 2012;13(9):869‐78. - PubMed
Klemenz 1997 {published data only}
-
- Klemenz BE. Subcutaneous versus intravenous rehydration therapy in older patients. Deutsche Medizinische Wochenschrift 1997;122(49):1540‐1. - PubMed
Koshy 2005 {published data only}
-
- Koshy RC, Kuriakose R, Sebastian P, Koshy C. Continuous morphine infusions for cancer pain in resource‐scarce environments: comparison of the subcutaneous and intravenous routes of administration. Journal of Pain & Palliative Care Pharmacotherapy 2005;19(1):27‐33. - PubMed
Lee 2009 {published data only}
-
- Lee Y, Koo J, Kim J, Park I, Joo M, Yoon J, et al. Effect of route of EPO administration on hemodialysis arteriovenous vascular access failure: a randomized controlled trial. American Journal of Kidney Diseases 2009;53(5):815‐22. - PubMed
Mace 2013 {published data only}
-
- Mace SE, Harb G, Friend K, Turpin R, Armstrong EP, Lebel F. Cost‐effectiveness of recombinant human hyaluronidase‐facilitated subcutaneous versus intravenous rehydration in children with mild to moderate dehydration. American Journal of Emergency Medicine 2013;31(6):928‐34. - PubMed
Mahalanabis 1970 {published data only}
Moulin 1991 {published data only}
-
- Moulin DE, Kreeft JH, Murray‐Parsons N, Bouquillon AI. Comparison of continuous subcutaneous and intravenous hydromorphone infusions for management of cancer pain. Lancet 1991;337(8739):465‐8. - PubMed
Nelson 1997 {published data only}
-
- Nelson KA, Glare PA, Walsh D, Groh ES. A prospective, within‐patient, crossover study of continuous intravenous and subcutaneous morphine for chronic cancer pain. Journal of Pain & Symptom Management 1997;13(5):262‐7. - PubMed
Paxton 2009 {published data only}
-
- Paxton JH, Knuth TE, Klausner HA. Proximal humerus intraosseous infusion: a preferred emergency venous access. Journal of Trauma 2009;67(3):606‐11. - PubMed
Rajani 2011 {published data only}
-
- Rajani AK, Chitkara R, Oehlert J, Halamek LP. Comparison of umbilical venous and intraosseous access during simulated neonatal resuscitation. Pediatrics 2011;128(4):954‐8. - PubMed
Ransome‐Kuti 1969 {published data only}
Robinson 1993 {published data only}
Soremekun 2009 {published data only}
-
- Soremekun OA, Shear ML, Patel S, Kim GJ, Biddinger PD, Parry BA, et al. Rapid vascular glucose uptake via enzyme‐assisted subcutaneous infusion: enzyme‐assisted subcutaneous infusion access study. American Journal of Emergency Medicine 2009;27(9):1072‐80. - PubMed
Tighe 1993 {published data only}
-
- Tighe SQ, Rudland SV, Kemp PM, Kershaw CR. Paediatric resuscitation in adverse circumstances: a comparison of three routes of systemic access. Journal of the Royal Naval Medical Service 1993;79(2):75‐9. - PubMed
Additional references
Deeks 2011
-
- Deeks JJ, Higgins JPT, Altman DG on behalf of the CSMG. Chapter 9: Analysing data and undertaking meta‐analyses. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Feldmann 2011
Fowler 2014
-
- Fowler RA, Fletcher T, Fischer WA II, Lamontagne F, Jacob S, Brett‐Major D, et al. Caring for critically ill patients with ebola virus disease. Perspectives from West Africa. American Journal of Respiratory and Critical Care Medicine 2014;190(7):733‐7. - PubMed
GRADEpro 2014 [Computer program]
-
- McMaster University. GRADEpro. Hamilton, Ontario: McMaster University, 2014.
Higgins 2011a
-
- Higgins JPT, Altman DG, Sterne JAC on behalf of the CSMG and the CBMG. Chapter 8: Assessing risk of bias in included studies. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Higgins 2011b
-
- Higgins JPT, Deeks JJ, Altman DG on behalf of the CSMG. Chapter 16: Special topics in statistics. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Hozo 2005
Kreuels 2014
-
- Kreuels B, Wichmann D, Emmerich P, Schmidt‐Chanasit J, Heer G, Kluge S, et al. A case of severe Ebola virus infection complicated by Gram‐negative septicemia. New England Journal of Medicine 2014 Oct 22 [Epub ahead of print]. [DOI: 10.1056/NEJMoa1411677] - PubMed
Lefebvre 2011
-
- Lefebvre C, Manheimer E, Glanville J. Chapter 6: Searching for studies. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Remington 2007
-
- Remington R, Hultman T. Hypodermoclysis to treat dehydration: a review of the evidence. Journal of the American Geriatrics Society 2007;55:2051‐5. - PubMed
Ribner 2014
-
- Ribner B. Ebola: Lessons Learned, from IDWeek 2014. http://www.idweek.org/ebola_idweek_2014/ (accessed 24 October 2014).
Rochon 1997
-
- Rochon PA, Gill SS, Litner J, Fischbach M, Goodison AJ, Gordon M. A systematic review of the evidence for hypodermoclysis to treat dehydration in older people. Journals of Gerontology. Series A, Biological Sciences and Medical Sciences 1997;52(3):M169‐76. - PubMed
Rouhani 2011
-
- Rouhani S, Meloney L, Ahn R, Nelson BD, Burke TF. Alternative rehydration methods: a systematic review and lessons for resource‐limited care. Pediatrics 2011;127(3):748‐57. - PubMed
Sanchez 2006
-
- Sanchez A, Geisbert TW, Feldmann H. Filoviridae: Marburg and Ebola viruses. In: Knipe DM, Howley PM editor(s). Fields Virology. 5th Edition. Philadelphia: Lippincott Williams & Wilkins, 2006:1409–48.
Schieffelin 2014
Schünemann 2011
-
- Schünemann HJ, Oxman AD, Vist GE, Higgins JPT, Deeks JJ, Glasziou P, et al. Chapter 12: Interpreting results and drawing conclusions. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
TSA ‐ Trial Sequential Analysis 0.9 Beta [Computer program]
-
- Copenhagen Trial Unit, Center for Clinical Intervention Research, Rigshospitalet. TSA ‐ Trial Sequential Analysis 0.9 Beta. Copenhagen: Copenhagen Trial Unit, Center for Clinical Intervention Research, Rigshospitalet, 2011.
Waitt 2004
WHO 2014
-
- World Health Organization. Ebola virus disease: Fact sheet number 103 (updated September 2014). http://www.who.int/mediacentre/factsheets/fs103/en/ (accessed 24 October 2014).
References to other published versions of this review
Ker 2014
-
- Ker K, Tansley G, Beecher D, Perner A, Shakur H, Harris T, Roberts I. Comparison of routes for achieving parenteral access with a focus on the management of patients with Ebola Virus Disease. Cochrane Database of Systematic Reviews 2014, Issue 11. [DOI: 10.1002/14651858.CD011386] - DOI - PMC - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

