Extracellular adenosine mediates a systemic metabolic switch during immune response
- PMID: 25915062
- PMCID: PMC4411001
- DOI: 10.1371/journal.pbio.1002135
Extracellular adenosine mediates a systemic metabolic switch during immune response
Abstract
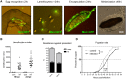
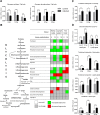
Immune defense is energetically costly, and thus an effective response requires metabolic adaptation of the organism to reallocate energy from storage, growth, and development towards the immune system. We employ the natural infection of Drosophila with a parasitoid wasp to study energy regulation during immune response. To combat the invasion, the host must produce specialized immune cells (lamellocytes) that destroy the parasitoid egg. We show that a significant portion of nutrients are allocated to differentiating lamellocytes when they would otherwise be used for development. This systemic metabolic switch is mediated by extracellular adenosine released from immune cells. The switch is crucial for an effective immune response. Preventing adenosine transport from immune cells or blocking adenosine receptor precludes the metabolic switch and the deceleration of development, dramatically reducing host resistance. Adenosine thus serves as a signal that the "selfish" immune cells send during infection to secure more energy at the expense of other tissues.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures





Comment in
-
Adenosine signaling and the energetic costs of induced immunity.PLoS Biol. 2015 Apr 27;13(4):e1002136. doi: 10.1371/journal.pbio.1002136. eCollection 2015 Apr. PLoS Biol. 2015. PMID: 25915419 Free PMC article.
References
-
- Pfeiffer T, Schuster S, Bonhoeffer S. Cooperation and competition in the evolution of ATP-producing pathways. Science. 2001;292: 504–507. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

