Photosynthetic Membranes of Synechocystis or Plants Convert Sunlight to Photocurrent through Different Pathways due to Different Architectures
- PMID: 25915422
- PMCID: PMC4411099
- DOI: 10.1371/journal.pone.0122616
Photosynthetic Membranes of Synechocystis or Plants Convert Sunlight to Photocurrent through Different Pathways due to Different Architectures
Abstract
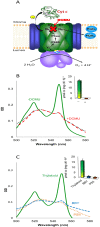
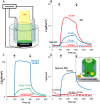
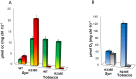
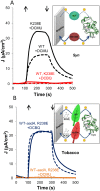
Thylakoid membranes contain the redox active complexes catalyzing the light-dependent reactions of photosynthesis in cyanobacteria, algae and plants. Crude thylakoid membranes or purified photosystems from different organisms have previously been utilized for generation of electrical power and/or fuels. Here we investigate the electron transferability from thylakoid preparations from plants or the cyanobacterium Synechocystis. We show that upon illumination, crude Synechocystis thylakoids can reduce cytochrome c. In addition, this crude preparation can transfer electrons to a graphite electrode, producing an unmediated photocurrent of 15 μA/cm2. Photocurrent could be obtained in the presence of the PSII inhibitor DCMU, indicating that the source of electrons is QA, the primary Photosystem II acceptor. In contrast, thylakoids purified from plants could not reduce cyt c, nor produced a photocurrent in the photocell in the presence of DCMU. The production of significant photocurrent (100 μA/cm2) from plant thylakoids required the addition of the soluble electron mediator DCBQ. Furthermore, we demonstrate that use of crude thylakoids from the D1-K238E mutant in Synechocystis resulted in improved electron transferability, increasing the direct photocurrent to 35 μA/cm2. Applying the analogous mutation to tobacco plants did not achieve an equivalent effect. While electron abstraction from crude thylakoids of cyanobacteria or plants is feasible, we conclude that the site of the abstraction of the electrons from the thylakoids, the architecture of the thylakoid preparations influence the site of the electron abstraction, as well as the transfer pathway to the electrode. This dictates the use of different strategies for production of sustainable electrical current from photosynthetic thylakoid membranes of cyanobacteria or higher plants.
Conflict of interest statement
Figures
Similar articles
-
The Photosystem II D1-K238E mutation enhances electrical current production using cyanobacterial thylakoid membranes in a bio-photoelectrochemical cell.Photosynth Res. 2015 Oct;126(1):161-9. doi: 10.1007/s11120-015-0075-3. Epub 2015 Jan 15. Photosynth Res. 2015. PMID: 25588957
-
Model quantification of the light-induced thylakoid membrane processes in Synechocystis sp. PCC 6803 in vivo and after exposure to radioactive irradiation.Photosynth Res. 2020 Dec;146(1-3):259-278. doi: 10.1007/s11120-020-00774-3. Epub 2020 Jul 30. Photosynth Res. 2020. PMID: 32734447
-
Spectrally decomposed dark-to-light transitions in Synechocystis sp. PCC 6803.Photosynth Res. 2018 Aug;137(2):307-320. doi: 10.1007/s11120-018-0505-0. Epub 2018 Mar 29. Photosynth Res. 2018. PMID: 29600442
-
Cytochrome b 6 f function and localization, phosphorylation state of thylakoid membrane proteins and consequences on cyclic electron flow.Photosynth Res. 2016 Sep;129(3):307-20. doi: 10.1007/s11120-016-0298-y. Epub 2016 Aug 17. Photosynth Res. 2016. PMID: 27534565 Review.
-
Photoprotection of photosystems in fluctuating light intensities.J Exp Bot. 2015 May;66(9):2427-36. doi: 10.1093/jxb/eru463. Epub 2014 Dec 1. J Exp Bot. 2015. PMID: 25468932 Review.
Cited by
-
NADPH performs mediated electron transfer in cyanobacterial-driven bio-photoelectrochemical cells.iScience. 2020 Dec 4;24(1):101892. doi: 10.1016/j.isci.2020.101892. eCollection 2021 Jan 22. iScience. 2020. PMID: 33364581 Free PMC article.
-
Hybrid bio-photo-electro-chemical cells for solar water splitting.Nat Commun. 2016 Aug 23;7:12552. doi: 10.1038/ncomms12552. Nat Commun. 2016. PMID: 27550091 Free PMC article.
-
Quantum Dots Assembled with Photosynthetic Antennae on a Carbon Nanotube Platform: A Nanohybrid for the Enhancement of Light Energy Harvesting.ACS Omega. 2023 Oct 26;8(44):41991-42003. doi: 10.1021/acsomega.3c07673. eCollection 2023 Nov 7. ACS Omega. 2023. PMID: 37969970 Free PMC article.
-
The Interaction of Water-Soluble Nitroxide Radicals with Photosystem II.Appl Magn Reson. 2022;53(7-9):1053-1067. doi: 10.1007/s00723-021-01425-z. Epub 2021 Sep 9. Appl Magn Reson. 2022. PMID: 34522067 Free PMC article.
-
The Development of Biophotovoltaic Systems for Power Generation and Biological Analysis.ChemElectroChem. 2019 Oct 31;6(21):5375-5386. doi: 10.1002/celc.201900997. Epub 2019 Sep 18. ChemElectroChem. 2019. PMID: 31867153 Free PMC article. Review.
References
-
- Blankenship RE (2014) Molecular Mechanisms in Photosynthesis. Chichester, UK: Wiley-Blackwell; 312 p.
-
- Nelson N, Ben-Shem A (2004) The complex architecture of oxygenic photosynthesis. Nat Rev Mol Cell Biol 5: 971–982. - PubMed
-
- Nelson N, Yocum CF (2006) Structure and function of photosystems I and II. Annu Rev Plant Biol 57: 521–565. - PubMed
-
- Sakurai I, Shen J-R, Leng J, Ohashi S, Kobayashi M, Wada H, et al. (2006) Lipids in oxygen-evolving photosystem II complexes of cyanobacteria and higher plants. Journal of biochemistry 140: 201–209. - PubMed
-
- Nelson N, Ben‐Shem A (2005) The structure of photosystem I and evolution of photosynthesis. Bioessays 27: 914–922. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

